Kentucky Pesticide Safety Education Program

Questions?
Contact
Dr. Ric Bessin
Dept. of Entomology
University of Kentucky
859-257-7450
rbessin@email.uky.edu
Source: Parts modified from Some Principles of Fungicide Resistance (PPFS-MISC-02) by Paul Vincelli, University of Kentucky Extension Plant Pathologist
Pesticide resistance presents an increasing challenge to growers. A resistant pest is one that is no longer controlled by a pesticide that has been effective in the past. Using the highest labeled rate and the minimum waiting period between applications does not improve control. Resistance is not limited to a single product or active ingredient. It occurs with all products that belong to the same pesticide class, that is, that have the same mode of action. The mode of action is the way the pesticide controls the pest. New labeling practices on some products make it easy to identify the modes of action of some products.
Sources of Resistance
Pesticides are important tools in pest management. Unfortunately, one of the risks of using them is that populations of resistant pests may develop. Thus products may become less effective - or even useless for controlling resistant pathogens and pests.
Resistance can only develop in pest populations where there is the genetic potential to resist the pesticide. Normally, only extremely low numbers of resistant individuals are present: perhaps many fewer than 1 in a million, but that can be enough to start the process. Usually, many pests are killed when a pesticide is applied. However, a few resistant individuals survive along with some susceptible ones that "escaped" the treatment, perhaps from incomplete spray coverage. The percentage of resistant individuals will be higher in the next generation. Each time the same pesticide is applied, the percentage of resistant individuals increases in the population and control decreases.
Development of resistant populations is a form of evolution resulting from genetic variability: some individuals happen to be resistant and this resistance has a genetic component that can be passed to the next generation.The genetic potential is largely out of human control. The mutation either exists in the field or does not.
The second factor is selection. Repeated use of the pesticide selects for individuals that can survive the presence of the toxin. That condition is under human control. It is a natural outcome of the use of at-risk pesticides. At-risk pesticides are those against which resistance is most likely to develop.
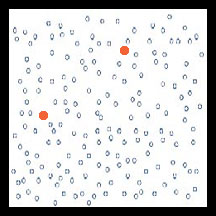 A population of pests (circles) before pesticide use. Most are susceptible (open circles) to the pesticide but a very low number are genetically resistant to the pesticide (filled circles).
A population of pests (circles) before pesticide use. Most are susceptible (open circles) to the pesticide but a very low number are genetically resistant to the pesticide (filled circles).
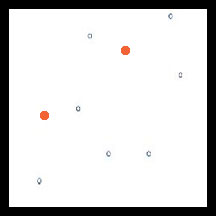 The resistant individuals survived. A few sensitive one (open circles) escaped the treatment, perhaps due to some factor like reduced spray coverage.
The resistant individuals survived. A few sensitive one (open circles) escaped the treatment, perhaps due to some factor like reduced spray coverage.
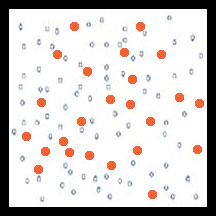 Under favorable conditions, the next pest generation will have a higher percentage of resistant individuals. Continued use of the pesticide selects for more resistant individuals. The labeled rate of the pesticide no longer is effective so a control failure occurs.
Under favorable conditions, the next pest generation will have a higher percentage of resistant individuals. Continued use of the pesticide selects for more resistant individuals. The labeled rate of the pesticide no longer is effective so a control failure occurs.
[return]
Herbicides
Herbicide resistance can develop from natural weed populations that are continuously exposed to herbicides that have the same mode of action. Kentucky has a relatively low number of documented cases but resistance is suspected in marestail, common ragweed, waterhemp, and palmer amaranth. Populations of smooth pigweed are resistant to triazine herbicides (i.e. Atrazine and Princep) in some areas of Kentucky where corn is grown in consecutive years.

Marestail and Palmer amaranth (www.foragefax.tamu.edu and www.ecosalon.com)
Glyphosate-resistant marestail can be found in most counties west of I-65 and is spreading eastward. It can emerge in the fall or overwinter or from early March through the summer. Glyphosate-resistant Palmer amaranth and waterhemp may be present in as many as 19 western Kentucky counties. They were first observed in fields located in flood plains or river bottoms but are now appearing in upland fields.
The common rotation of 3 crops over a 2-year period contributes to the relatively few documented cases of resistance in the state. Corn is planted in the spring of the first year, followed by fall-planted wheat. Soybeans are planted the second year in early to mid-June after wheat harvest. This rotation accounts for about 1/4 of the soybean, 1/3 of the corn, and nearly 3/4 of the state's wheat acres.
Herbicide resistance can be intentional. For example, soybean varieties and corn hybrids have been developed that are resistant to glyphosate products. However, this can result in glyphosate-resistant corn increasing either as volunteer plants or as unwanted stands in replanting situations.
Prevention is a key to avoiding development of herbicide resistant weed populations. Here are management strategies to consider in preventing and dealing with herbicide resistant weeds:
![]() Scout fields regularly and identify weeds present. Respond quickly to shifts in weed populations to restrict spread of weeds.
Scout fields regularly and identify weeds present. Respond quickly to shifts in weed populations to restrict spread of weeds.
![]() Select a herbicide based on weeds present and use a herbicide only when necessary.
Select a herbicide based on weeds present and use a herbicide only when necessary.
![]() Rotate crops. Crop rotation helps disrupt weed cycles and some weed problems are more easily managed in some crops than others.
Rotate crops. Crop rotation helps disrupt weed cycles and some weed problems are more easily managed in some crops than others.
![]() Rotate herbicides. Avoid using the same herbicide or another herbicide with the same mode of action (i.e. herbicides that inhibit the same process in target weeds) for two consecutive years in a field.
Rotate herbicides. Avoid using the same herbicide or another herbicide with the same mode of action (i.e. herbicides that inhibit the same process in target weeds) for two consecutive years in a field.
It is possible for a herbicide used in one crop to have the same mode of action as a different herbicide used in another crop. For example: Accent, Classic, Harmony Extra, Harmony SG, Lightning, Scepter, Option, Osprey, Permit, Pursuit, Spirit, Python, Resolve, Steadfast, Stout, and Synchrony “STS” contain active ingredients with the same mode of activity in plants (i.e. these herbicides are ALS/AHAS inhibitors).
Herbicide group numbers help to identify modes of action. Group 2 herbicides are ALS/AHAS inhibitors.
![]()
![]() Apply herbicides with different modes of action as a tank mixture or sequential application during the same season.
Apply herbicides with different modes of action as a tank mixture or sequential application during the same season.
![]() Combine other weed control practices such as cultivation with herbicide treatments where soil erosion potential is minimized.
Combine other weed control practices such as cultivation with herbicide treatments where soil erosion potential is minimized.
![]() Clean tillage and harvest equipment to avoid moving weed problems from one field to the next.
Clean tillage and harvest equipment to avoid moving weed problems from one field to the next.
[return]
Fungicides
A diversified plant disease management program will slow down the development of fungicide resistance. Furthermore, even if resistance develops, it will not be as damaging, as compared to a farm where only fungicides are used for disease control. A diversified plant disease management program is buffered against severe damage from fungicide-resistant strains, since there are other tactics that are contributing to disease management. The best way to protect the utility of fungicides is by not over-relying on them. Many crop-management practices can help reduce the reliance on fungicides.
Strategies for managing fungicide resistance are aimed at delaying its development and limiting crop losses if resistance develops. A management strategy should be in place before resistance becomes a problem.
Appropriate disease-control practices may include:
![]() Crop rotation
Crop rotation
![]() Resistant varieties
Resistant varieties
![]() Management of irrigation and leaf surface moisture
Management of irrigation and leaf surface moisture
![]() Fertility practices that impact disease
Fertility practices that impact disease
![]() Planting dates that reduce disease risk
Planting dates that reduce disease risk
![]() Sanitation in all its many forms
Sanitation in all its many forms
![]() Plant spacing and sowing practices that reduce disease
Plant spacing and sowing practices that reduce disease
![]() Management of vectors and other pests
Management of vectors and other pests
![]() Improved surface and subsurface drainage
Improved surface and subsurface drainage
![]() Raised beds
Raised beds
![]() Cover crops that reduce disease pressure
Cover crops that reduce disease pressure
![]() Addition of organic matter to soil
Addition of organic matter to soil
![]() Mulching
Mulching
![]() Pathogen-free seed
Pathogen-free seed
How Fungicide Resistance Can Develop
The use of fungicides increases the risk of resistance. Anytime a fungus is exposed to a fungicide, even if fungal activity is low, the selection pressure is increased towards resistance. Resistance to strobilurin fungicides is a worldwide concern because these fungicides are known for being prone to resistance development.

Frog eye leaf spot of soybean (www.agproduction.com)
Strobilurin-resistant isolates of Cercospora sojina, the cause of frogeye leaf spot of soybean, have been found in several states, including Kentucky. This is a "warning shot" when it comes to strobilurin use for both soybean and corn. Specifically, the widespread occurrence of strobilurin-resistant C. sojina in a field in west Tennessee shows that resistance can develop in field crops in response to overuse in a production setting.
The DMI or triazoles family of fungicides, commonly used on corn, also are prone to the development of fungal resistance. For decades, scientists have watched as fungi all over the world have become incrementally more resistant to DMI fungicides. The use of any fungicide for "plant health" reasons increases the risk of developing resistance. Other than never using them, there is no way to prevent resistance to strobilurins and DMI fungicides.
The only hope is to slow down resistance development. The best way to do that is to minimize the use of the at-risk fungicides. Factors that increase the potential for fungicide resistance include:
- Over-use or repeated applications of fungicides with the same chemistry, alone or in mixes with other fungicides.
- Applying fungicides at half-rates. Using lower than label rates of DMI fungicides will not killing all the target pathogens. Those that survive are likely to be less sensitive to the fungicide the next time it is applied. In the case of strobilurin fungicides, resistance development is usually not impacted by application rate and can occur equally at low or high rates of application.
- Applying a pesticide when disease pressure is already high. A field that has been severely damaged by disease cannot be cured and there is a good chance that surviving target organisms could result in the development of resistance.
The only way to absolutely prevent resistance is to not use a fungicide that can cause resistance to develop. This is not practical because many currently-used fungicides that provide highly effective, broad-spectrum disease control are at risk for resistance.
Mode of action group and resistance management strategies are now clearly included on the registration labels of most site-specific fungicides. These allow you to identify mode of action groups for rotation programs.
[return]
Insecticides
IRAC numbers help you to identify the mode of action of insecticides. Products with the same group number attack the same target in the pest. Rotation among products with different modes of action delay development of resistant populations. Repeated use of products in the same group selects for resistant individuals.
Insecticide group numbers appear on some labels making it easy to rotate modes of action to reduce the potential for development of resistant populations. For example, pyrethroid insecticides belong to Group 3. Continued use of insecticides in this group can lead to resistance. Rotate with other numbered groups to manage potential resistance problems. Insecticide resistance can develop rapidly in populations of arthropods with short life cycles (aphids and mites) living in closed production situations like greenhouses. The potential for resistance to Bt toxins in corn pests, such as the European corn corer, is high because of the large percentage of Bt corn acreage planted each year.
![]()
IRAC code numbers appear on the front of newer insecticide labels to identify insecticide mode of action groups (www.msue.anr.edu)
Managing Resistance in Bt Corn
Bt corn hybrids have been modified to contain a gene from the soil bacterium Bacillus thuringiensis (Bt) that codes for production of insect-specific toxins in corn tissue. Lepidoptera Bt strains are specific for caterpillars (European corn borer, cutworms, armyworms, etc.) while others kill corn rootworm larvae. Widespread adoption of Bt varieties has increased the number of acres where pests are exposed to the Bt active ingredient. With much of the pest population exposed to Bt toxins every season, resistant pest populations can develop through a selection process.
An insect resistance management (IRM) plan was developed to prolong the effectiveness of Bt crops. The initial IRM strategy was to prevent or delay resistance to Bt varieties by planting refuge acres of the crop on each farm that do not have have the trait used in the Bt planting. The refuge needed to be 20% of the corn acreage on each farm with specific arrangement and distance requirements. The goal of a refuge is to produce target pests that are not exposed to the Bt toxin. These Bt-susceptible individuals would mate with potentially resistant individuals that developed on the plants with the Bt gene. Matings between susceptible and resistant individuals keeps Bt-susceptibility in the pest population.
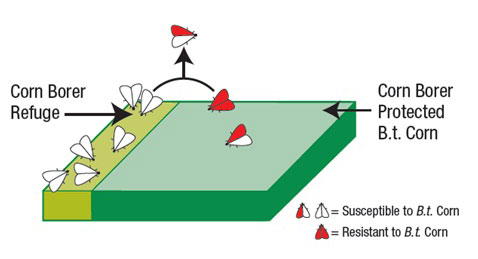
The goal of a refuge is to produce susceptible individuals to mate with resistant individuals. (www3.syngenta.com)
- Refuges for the Lepidoptera-protected Bt corn (European corn borer, cutworm, armyworm) must be 20% of the total corn acreage on the farm, within 1/2 mile of the Bt-corn, and managed by the same grower.
- Refuges of Bt rootworm-protected corn has the same acreage requirements but must be within or directly next to the Bt rootworm field due differences in behavior of the insects.
The planting of refuges is required by the EPA in order to allow the registration and sale of Bt-crops. Farmers who grow Bt-crops for 2 consecutive years without planting required refuges will not be allowed to grow a Bt crop in the third year. The EPA may cancel the registration of crops with Bt traits if farmers are largely not complying with the requirement. The EPA requires that companies that register Bt corn identify and address non-compliant farmers through field and planting record inspections. (Source: http://corn.agronomy.wisc.edu/Management/pdfs/A3857.pdf)
[return]
1) A resistant pest is one that is no longer controlled by a pesticide that was effective in the past.
2) Pesticide resistance is limited to a single active ingredient, all other pesticides with the same mode of action are still effective.3) Pesticide resistance has a genetic basis; the trait is passed along to the offspring of resistant individuals.
4) Development of resistance is a selection process resulting from _______ in pest populations.
5) Resistance is most likely to develop against _________ pesticides.
6) Relatively few documented cases of herbicide resistance have developed in Kentucky grain crops because of _____________.
- low pesticide use
- heavy reliance on non-chemical control
- wide use of a corn-wheat-soybean rotation
- avoiding pesticide rotations
7) Prevention is the key to avoiding pesticide resistance
8) Using the same pesticide or a different brand of pesticide with the same mode of action for 2 or more consecutive years _______ the chances of problems with pesticide resistance.
9) Using a combination of chemical and non-chemical pest control practices is an effective way to reduce development of pesticide resistance.
10) It is best to use resistance management practices only after a problem develops.
11) Which of the following IS an appropriate disease control practice?
- Plant only varieties that are very susceptible to diseases
- Leave crop residues on the surface from one season to the next
- Use planting dates and plant populations that reduce disease
- Do not rotate crops to reduce disease
12) Using pesticides as "plant health" chemicals when no potentially damaging pest population is present ____________ the risk of developing resistance.
13) Applying a fungicide at half of the label rate is a generally recommended resistance management practice.
14) It is best to begin fungicide applications only after disease pressure is high.
15) The words "Group 3 Insecticide" on a label means that you can use the product for 3 seasons before rotating to a product with a different mode of action.
16) The widespread adoption of Bt-corn for caterpillar control ___________ the chances for the development of resistance.
17) The goal of a refuge in the resistance management strategy for Bt crops is to ____________________.
- reduce the need for extra fungicide applications
- produce Bt-susceptible individuals
- produce Bt-resistant individuals
18) The EPA ____________ the planting of refuges in order to allow the registration and sale of Bt-crops.
website designed by P.M. Dillon copyright © 2015 University of Kentucky Department of Entomology
University of Kentucky College of Agriculture |
S-225 Agricultural Science Center North, Lexington, KY 40546-0091 | 859.257.7450
An Equal Opportunity University |
Last modified
11/30/2018
