
 The third distinct moth flight of the season was recorded in Princeton last week. This means that the third generation
larvae will soon be attacking corn.
The third distinct moth flight of the season was recorded in Princeton last week. This means that the third generation
larvae will soon be attacking corn.


 The third distinct moth flight of the season was recorded in Princeton last week. This means that the third generation
larvae will soon be attacking corn.
The third distinct moth flight of the season was recorded in Princeton last week. This means that the third generation
larvae will soon be attacking corn.
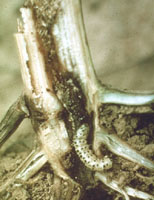
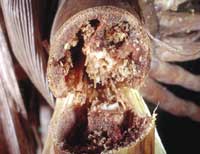 It is this generation that will move to the bottom of the plant to overwinter.
And it is
these same larvae that girdle the stalk one to two inches above the soil line in September. Later planted fields will
be more attractive for egg laying, so they will be more likely to be damaged. In parts of the state where only two
generations occur, the overwintering second-generation larvae do the same type of damage.
It is this generation that will move to the bottom of the plant to overwinter.
And it is
these same larvae that girdle the stalk one to two inches above the soil line in September. Later planted fields will
be more attractive for egg laying, so they will be more likely to be damaged. In parts of the state where only two
generations occur, the overwintering second-generation larvae do the same type of damage.
At this point, what can we do to control the larvae? Not much. Fields that were planted with full-season-control Bt hybrids should not see much damage. Insecticidal control at this point would not be a good decision, but identifying fields with the worst infestations and selecting them for the earliest reasonable harvest would be a good decision.
For more information about corn pests, visit
"Insect Management Recommendations".
 Several late-season diseases are showing up in early
planted corn.
Several late-season diseases are showing up in early
planted corn.
Diplodia ear rot (caused primarily by the fungus Stenocarpella maydis) was diagnosed last week in a field in the Green River Extension Area, and may be showing up in other locations throughout the state. In healthy corn, the husks dry from the tip downward, Ears infected by S. maydis often turn straw-colored at the base, or the entire husk may be prematurely dried. Strip away the husks and you'll see a white mold growing between the kernels, usually progressing from the base of the ear but soon consuming the entire ear. One may also see individual leaf blades dry prematurely from infections of the leaf sheath.
Since S. maydis survives in corn residue, risk factors for the disease include lack of rotation and conservation tillage practices. Certain hybrids appear to be highly susceptible, although information on hybrid susceptibility to this disease is usually not included in seed catalogs. Since some companies do not deliberately screen their hybrids against Diplodia ear rot, some seed suppliers rely on general field observations as a guide to hybrid susceptibility to this disease, which is a weak guideline at best. Nevertheless, avoid hybrids with a history of the disease in your area, and rotate away from corn in no- till fields where more than 2-3% of ears are showing the disease this season. For more information, see the UK Extension Publication, "Ear Rot of Corn Caused by Stenocarpella maydis" , PPA-43.
Stalk rot diseases were moderately severe in a field visited last week in the Pennyrile Extension Area in southwestern Kentucky. The principal disease found was charcoal rot, although some plants appeared to have Fusarium stalk rot. Look for prematurely dried plants whose lower stalk crushes easily when squeezed between thumb and forefinger. When cut open, the pith of stalks affected by charcoal rot will have a gray appearance, due to the presence of thousands of tiny round black survival bodies called "sclerotia". These are easily seen with a hand lens. Fusarium stalk rot usually produces no distinctive symptoms or signs in stalks. A tentative field diagnosis can be based on the presence of prematurely dried plants having soft lower stalks without evidence of other stalk rot diseases. Affected plants may exhibit a whitish moldy growth on the outside of affected stalk tissues.
According to weather maps from the UK Agricultural Weather Center, many of the southern-tier counties had received very little rainfall in the last six weeks until the rains of this past weekend. Drought during grain fill is a major predisposing factor for charcoal rot (which also can attack soybean), and Fusarium stalk rot is generally of greatest concern in warm, dry conditions. Fields that received rainfall this past weekend are at reduced risk from these stalk rots, but many of the plants in fields that experienced drought this summer are probably already infected. Thus, it would be wise to scout for stalk rots as these crops approach maturity. An easy way is to walk through the field and push stalks 8-10" from vertical at about chest height. Stalks with stalk rots or reduced stalk strength for other reasons will fall. If 10-15% of the field exhibits reduced stalk strength, consider early harvest for the field, so as to avoid losses due to lodging. More information can be found in the UK Extension publication, "Corn Stalk Rots".
![]() Stem canker (SC) and sudden death syndrome (SDS)
will soon be visible in many full-season soybean
fields. Both of these diseases tend to kick into high
gear about the mid pod fill stages. Because of the
media hype that tends to occur when SDS begins to
show up in fields, most farmers are generally aware of
the symptoms and ramifications of that disease. In
contrast, SC, which is a much more significant threat
to soybean production in Kentucky, in most years, has
been given little press. This lack of attention is due to
the fact that serious SC epidemics are few and far
between and because, superficially, the foliar
symptoms of SC resemble those of SDS. As a result, a
lot of SC is incorrectly identified as SDS.
Stem canker (SC) and sudden death syndrome (SDS)
will soon be visible in many full-season soybean
fields. Both of these diseases tend to kick into high
gear about the mid pod fill stages. Because of the
media hype that tends to occur when SDS begins to
show up in fields, most farmers are generally aware of
the symptoms and ramifications of that disease. In
contrast, SC, which is a much more significant threat
to soybean production in Kentucky, in most years, has
been given little press. This lack of attention is due to
the fact that serious SC epidemics are few and far
between and because, superficially, the foliar
symptoms of SC resemble those of SDS. As a result, a
lot of SC is incorrectly identified as SDS.
Once you know all of the symptoms associated with both SDS and SC, differentiation of the two diseases becomes relatively simple.
Root symptoms:
SDS: Primary, secondary and tertiary roots are
severely rotted. Nitrogen-fixing nodules are
mushy.
SC: Roots healthy.
Stem symptoms:
SDS: Exterior of stem appears healthy. Interior of
stem is a milky-brown color compared to the
yellow-white color of a healthy stem.
SC: Early symptoms are a dark brown-firm
canker in the vicinity of nodes which are visible
on the external stem. At this time, the interior of
the stem will be unaffected, except for slight
discoloration associated with the exterior canker.
As the disease progresses, the dark brown canker
may extend the length of the stem, often on one
side, but the entire stem may become involved.
Individual branches of plants may die, while
others remain unaffected. The interiors of severely
diseased stems are often completely deteriorated.
Foliar symptoms:
SDS: Yellow blotches form between the veins,
usually developing first on the uppermost leaves.
In a few days the yellow blotches will coalesce
and begin to turn brown. The end stage is
complete tissue death between the veins, with the
only green tissue remaining being that associated
with the primary leaf veins. The edges of severely
diseased leaves will roll inward. Over time the
diseased leaflets may fall off the petioles or they
may remain attached to the plant. Serious yield
loss usually only occurs when plants are
exhibiting serious foliar symptoms BEFORE mid
pod fill. After that time, plants can look pretty
rough, but yields may still be little affected.
SC: Unlike SDS which initially appears as yellow
spots, leaves of plants with SC develop a general
yellowing in the tissue between the veins. Over
time, these areas die and the end symptoms are
essentially identical to those caused by SDS. More
often than not, however, the leaves of plants with
SDS will drop off the plant while those with SC
will die and remain attached to the plant.
Time of appearance:
SDS and SC: Plants are infected early in the season, but symptoms are rarely (in the case of SDS) and never (in the case of SC) expressed prior to the plant reaching the reproductive stages.
Pattern of symptoms:
SDS: Individual and groups of plants, 10-50 feet
in radius, usually show a range of symptoms
ranging from some leaf spotting to complete
defoliation. Wet or otherwise stressed areas of
fields, such as long field edges, will usually be the
first to develop symptoms. In extreme cases,
entire fields may show symptoms. When SDS is
severe, symptoms will first develop in "hot spots"
and later progress into other areas. This gives the
effect that the disease spreading, but in reality it is
not. Rather the time of infection, crop health, and
field conditions vary, so disease symptoms are
expressed at varying times and rates.
SC: Single random plants in small to large areas
die seemingly overnight. It is not uncommon for
entire fields to be completely destroyed by SC in
more southern states. Fortunately, complete wipe-
outs due to SC are very rare in KY. Since plants
are infected only during the vegetative stages,
symptoms will not "spread" to unaffected plants
once they begin to appear during mid to late
season. Similar to SDS, SC may appear to spread
somewhat only because infection levels and rates
of symptom expression may vary from plant to
plant and field to field.

Rust has been very active on perennial ryegrass in some settings and may be active in athletic fields or other locations. A severe case was also found on a new seeding of kentucky bluegrass sod. Look for cinnamon brown pustules (each comprised of masses of hundreds or thousands of spores) on affected leaf blades. The tissue around the pustules may turn brown, and affected leaves eventually wither, turn light brown, and die. Rust has appeared earlier than I am accustomed to seeing, so we may see continued increases in disease activity over the next 6-8 weeks or so, when rust pressure is usually highest in Kentucky.
This disease is typically most severe on slowly growing turfs, especially those receiving limited water and/or nitrogen fertility. In most instances, a light application of a slow-release fertilizer (0.5 to 0.75 lb N/1000 sq ft) supplemented with irrigation as needed will be sufficient to help the turf outgrow the disease. Some such action is probably advisable in swards showing severe damage at this time, since this disease could impose a substantial stress on the grass should the weather turn hot and dry for an extended period later this summer. Severely affected swards are those where the leaf yellowing and rusty color can be easily seen in the grass at your feet from a standing height. Avoid putting on high amounts of quick-release nitrogen on perennial ryegrass at this time, since that can enhance susceptibility to other destructive diseases such as brown patch, Pythium blight, and gray leaf spot.
Fungicides may have a place in high-maintenance swards such as golf courses, if the disease is active. The fungicides that are known to be most effective against rust are the DMI fungicides propiconazole (Banner MAXX) and triadimefon (Bayleton, Proturf Fungicide VII). The contact fungicides chlorothalonil (Daconil, Thalonil, and other formulations) and mancozeb (Fore, etc) are also reasonably effective. Contacts must be applied before infections occur and can therefore only protect from new infections. The DMI fungicides may exhibit 24-48 hours of "kickback activity" by eradicating infections less than 48 hours old. However, any visible pustules are much older than that and therefore cannot be eradicated using these fungicides, either. Thus, expect fungicides to promote recovery by protecting the new growth rather than curing existing foliage.
As of 9:45 am on 31 Jul 00, I have no recorded instances of gray leaf spot on perennial ryegrass in Kentucky or the region. I have found substantial leaf spotting on perennial ryegrass due to the fungus Bipolaris sorokiniana. This fungus produces brown leaf spots that develop tan centers as they age. These leaf spots can be impossible to distinguish from gray leaf spot without a microscope. Thus, the presence of leaf spots at this time of year should be evaluated at a diagnostic lab; don't assume it is gray leaf spot without a proper evaluation.
The cool, dry weather of last week likely suppressed gray leaf spot activity for a time, but the warm, wet conditions of this past weekend were probably conducive for infection. Although levels of primary inoculum may be low because of last year's drought, the next six weeks are the period of highest risk from gray leaf spot, so managers of high-maintenance perennial ryegrass should have preventive spray programs in place at this time. Heritage at 0.4 oz, Cleary's 3336 at 6-8 oz, or Spectro 90DG at 8 oz (per 1000 sq ft) are highly effective choices. Compass at 0.20 oz or Banner MAXX (1 oz) plus Daconil Ultrex (3.7 oz) are also good choices. Of all these, the only treatment that can provide more than two weeks of control under high disease pressure is Heritage at 0.4 oz.
 With the need to diversify their crops many Kentucky
farmers have planted grapes. To successfully grow
grapes, growers need to be aware of grape diseases.
In previous newsletters, cane, foliage and fruit
diseases have been discussed. In the plant disease
diagnostic laboratory, grape specimens with crown
gall are now being observed, thus a discussion of this
important trunk disease is warranted. There are more
than 600 types of plants susceptible to crown gall
disease. Crown gall is especially devastating to
grapes in Kentucky and some vineyards have been
lost due to the disease, but it can also affect other
fruits such as apples, stone fruits, and brambles.
With the need to diversify their crops many Kentucky
farmers have planted grapes. To successfully grow
grapes, growers need to be aware of grape diseases.
In previous newsletters, cane, foliage and fruit
diseases have been discussed. In the plant disease
diagnostic laboratory, grape specimens with crown
gall are now being observed, thus a discussion of this
important trunk disease is warranted. There are more
than 600 types of plants susceptible to crown gall
disease. Crown gall is especially devastating to
grapes in Kentucky and some vineyards have been
lost due to the disease, but it can also affect other
fruits such as apples, stone fruits, and brambles.
Symptoms. The disease is characterized by galls or knobby overgrowths that form on susceptible plant tissues. New galls first appear in early summer as white, fleshy, callus growth. Galls turn brown by late summer and in the fall become dry and corky. The woody tumors may be gnarled with rough surfaces. Galls can develop rapidly and completely girdle a young vine in one season, or they may take a few years to develop. Galled vines frequently produce inferior shoot growth, and portions of the vine above the galls may die. When galls are numerous or when they are located on the major roots or on the root crown, they disrupt the translocation of water and mineral elements, leading to poor growth, gradual dieback, and sometimes death of vines. In general, affected plants are more susceptible to adverse environmental conditions, especially winter injury.
Cause and biology of the disease. Crown gall is caused by the soil-borne bacterium, Agrobacterium tumefaciens. The bacterium survives for long periods of time in soil, and also in galls and in diseased plants. The crown gall bacterium is widely present in Kentucky soils and may be systemically present in many grape vines, but seldom causes disease unless the vine is injured. Galls develop following an injury permitting entrance of the pathogen and may appear on the roots, trunk, and arms of grape vines. Such injuries may occur during intermittent freezing and thawing weather common to Kentucky each winter. Such frequent freezing and thawing may not occur in other grape growing regions such as New York or California. Overwintering bacteria may be spread to wound sites by splashing rain, running water, on cultivation implements or on pruning tools. Contaminated nursery stock may be another source of the disease. Bacteria can survive in the soil for many years.
Disease management.
Use disease tolerant cultivars. In general, Vitis vinifera grapes are more susceptible than V. labrusca. Highly susceptible cultivars include Baco Noir, Cabernet Franc, Cabernet Sauvignon, Chancellor, Chardonnay, Gewrtztraminer, Limberger, Merlot, Muscat Ottonel, Pinot Blanc, Pinot Gris, Pinot Meunier, Pinot Noir, Riesling, and Sauvignon Blanc. Less susceptible cultivars include Cascade, Catawba, Concord, Delaware, Einset Seedless, Foch, Fredonia, Ives, Steuben, Vanessa, and Ventura.
Select planting sites with no history of crown gall, or wait at least 5 years before replanting such sites. Plant vines in well drained soil. Minimize root injuries during planting. Using northeast facing sites may reduce freeze injury. Plant only certified, disease-free nursery stock; discard plants with galls. The biological control microbial antagonists used for crown gall at planting do not work for grapes. Soil fumigation is generally not effective for destroying the pathogen.
Adopt management practices that minimize wounding. Hill up soil or mulch around grapevines or otherwise protect the lower trunk in fall to reduce winter injury and resulting wound sites needed for infection. Hilling also ensures the development of new scion shoots that may be needed for trunk renewal. In some areas growers bury young vines in the fall to reduce freeze injury.
Generally, remove and destroy infected plants, however, galls on the upper parts of the trunk or on canes can sometimes be pruned out.
The double trunk system of training may be a useful system for minimizing losses due to crown gall. If one trunk is infected, it can be removed. The remaining trunk can be pruned leaving a full number of buds until the second trunk can be renewed.
Grape vines with poor vigor are more susceptible to winter injury, thus it is important to manage the crop and other grape diseases so as to insure maximum vine vigor.
 Many Kentucky farmers are growing pumpkins, but
few are paying adequate attention to timing the
needed disease controls. Pumpkins are highly prone
to a number of diseases in Kentucky, with August and
September the months of greatest disease activity.
This year, disease activity is occurring even earlier
and increasing faster than normal. The weather of
this past weekend did not help. Some growers are
just putting out the crop and making no attempts to
make timely applications of fungicides, bactericides,
and vector controlling insecticides. As a result, the
canopy has now closed in most plantings and diseases
are well established. Moreover, many experienced
growers are not following recommended practices of
disease control concerning rotation and field selection.
Many Kentucky farmers are growing pumpkins, but
few are paying adequate attention to timing the
needed disease controls. Pumpkins are highly prone
to a number of diseases in Kentucky, with August and
September the months of greatest disease activity.
This year, disease activity is occurring even earlier
and increasing faster than normal. The weather of
this past weekend did not help. Some growers are
just putting out the crop and making no attempts to
make timely applications of fungicides, bactericides,
and vector controlling insecticides. As a result, the
canopy has now closed in most plantings and diseases
are well established. Moreover, many experienced
growers are not following recommended practices of
disease control concerning rotation and field selection.
In a recent survey, the following issues were identified as deficiencies in the disease control program at the farm level.
* Failure to properly rotate - Plantings of fields where the disease potential has been allowed to decline by proper rotation to non-host crops was seldom being practiced. Instead, growers were using rotations known to increase disease potential such as pumpkins following pumpkins, other cucurbits, tobacco, peppers, tomatoes, or melons. In some sites, volunteer pumpkins were present in or around the planting.
* Poor site selection - Fields were often located in areas of very poor air circulation and with fog pockets, such as river bottoms. When one grower was questioned about his using this poor practice, he commented that this bottom is too wet most years for tobacco, but pumpkins did well there in 1999. Folks remember that in 1999, we experienced a 100-year record drought! This year is more typical of Kentucky's moisture patterns.
* More poor site selection - Pumpkins were planted adjacent to spring plantings of other cucurbits, a practice that almost insures that diseases building in the spring crop will attack the fall crop, even earlier than normal. When I ask one grower why he planted them adjacent to each other his response was: " It is easier to keep up with them". My response was: " Yes, and it is easier for more of the microbes to find your later plantings when they are adjacent."
* Inadequate spray equipment and timing of applications - Most pumpkin growers do not have adequate spray equipment. Pumpkins are a difficult crop to cover with fungicides. The canopy is thick, and increases rapidly, with the stems (runners) and young fruits hidden within this dense canopy. It takes frequent applications and pressure to get the fungicides onto those critical targets. Also, too many growers are waiting until serious crop damage is occurring before starting spray programs.
Below are some specific disease control issues that need attention:
Bacterial Wilt is already very active, yet most growers have not made a single vector-control spray of insecticide. Applications of insecticides are needed starting at emergence of the seedlings to control this disease. Cucumber beetle feeding was very evident on crown and runners in most fields checked. Folks, controlling cucumber beetles before feeding on the plant is essential for prevention of this disease!
Bacterial Leaf Spots activity has increased sharply during the past two weeks. This disease can cause significant damage to the foliage, but this activity should provide an abundance of inoculum for the fruit rot phase of this same disease. The disease was present to some level in most fields checked, but it was especially active on farms with a history of pumpkins. Control is centered around preventing the build up of bacteria on the foliage and by keeping the young fruit from becoming infected. Weekly sprays of copper-containing bactericides/fungicides are needed with the earliest symptoms, but no later than when fruits are forming to control the fruit phases of the disease.
Downy Mildew was not found in any field checked. However, downy mildew usually appears to some degree yearly in late summer (after August 15) causing tiny yellow spots on the leaves that quickly expand to blight the entire leaf, but it can develop earlier. The more broad spectrum fungicides (containing chlorothalonil, mancozeb, copper) will suppress development and should be in place now for the control of other diseases, as well. Stay alert for warnings and immediately shift to the better downy mildew controls should watches or warnings be issued.
Microdochium Blight was common in unsprayed fields. This is a newly recognized disease in the area, but probably has been present a long time. It occurs as white dashes, flecking, and etching (russeting) or ashy-like material on the stems and fruit surfaces. It is also causing a serious blight of foliage that could easily have been confused with gummy stem blight, resulting from petiole and leaf-vein cankers blighting leaves, and white-tan, spindle-shaped cankers girdling the main stems. Regular fungicide programs are needed to control this disease.
Powdery Mildew was found in shady areas of a few fields. This is the most important disease of late summer and fall cucurbits in Kentucky. Its control is critical to successful production in Kentucky nearly every year. Fungicides that are systemic or have translaminar activity are needed to obtain adequate protection of the underleaf surfaces, where conditions are more favorable for development of the pathogen than on upper surfaces. Because the fungicides that have this type activity have single-site modes-of- action, the mildew fungi develop resistance to them. Therefore, fungicide-resistance management is needed on each and every field. The powdery mildewcides should be applied in ways to reduce the risk of resistance developing. The regular fungicide program of broad spectrum fungicides, such as Chlorothalonil, or Mancozeb, used for control of other diseases should be modified to include in either a tank mix or alternation (as per each label) a powdery- mildewcides - strobilurin fungicides (Flint or Quadris), DMI-fungicides (Nova or Bayleton), or benzimidazoles (Benlate or Topsin ). Do not select one of these and go all season.
Virus Complex is active in most fields. Cucumber Mosaic Virus and Watermelon Mosaic Virus are both very active and at higher levels for this time of the season. No controls are available for them at this stage of the crop.
 With early summer vegetable production winding
down, now is the time to clean up these fields for next
year. Early sanitation at this time of the year can (1)
cause adult insect pests to move out of the field and
(2) eliminate the food source for immature pests so
that they cannot complete their life cycle. Keep in
mind, that although it may not be profitable to
continue to harvest some vegetables because of the
low price in the market of the poor quality of this tail-
end produce, it is still attractive to pests. Many of
these pests that develop at the end of the season will
be the early colonizers of fields next spring.
With early summer vegetable production winding
down, now is the time to clean up these fields for next
year. Early sanitation at this time of the year can (1)
cause adult insect pests to move out of the field and
(2) eliminate the food source for immature pests so
that they cannot complete their life cycle. Keep in
mind, that although it may not be profitable to
continue to harvest some vegetables because of the
low price in the market of the poor quality of this tail-
end produce, it is still attractive to pests. Many of
these pests that develop at the end of the season will
be the early colonizers of fields next spring.
Several of the more serious insect pests such as European corn borer, squash vine borer, Mexican bean beetle, squash bug, diamondback moth, tobacco and tomato hornworm, cabbage looper, and imported cabbageworm are able to continue development on crop residues in the garden long after we take what we consider the edible vegetables. Other pests such as flea beetles can find food and shelter from weeds as well as crop residues throughout the winter. The two-spotted spider mites continue to feed on weeds after the crops have withered.
A thorough fall cleanup should help to discourage some of the pests that may cause problems next year. Commercially, fields can be disked to destroy crop residues. Home gardeners can compost or till these residues into the soil. It is important to keep in mind that this should not be just a fall practice to destroy crop residues, as soon as a crop has been harvested for the last time, clean up should begin, even if that is early summer for spring crops.

Samples on field crops and forages last week included rust, Diplodia ear rot and gray leaf spot on corn; Rhizoctonia stem canker on alfalfa; brown stripe on orchardgrass; rust and loose smut on fescue; Phytophthora blight, Rhizoctonia and Fusarium stem rots on soybean; black root rot, black shank, soreshin, Fusarium wilt, target spot, blue mold, virus complex and damage from sucker control chemicals on tobacco.
On fruits and vegetables, we have seen Septoria leaf spot and Verticillium wilt on blackberry; crown gall on grape; bitter rot, cedar apple rust, and frogeye on apple; cork spot on pear; bacterial wilt on cantaloupe; angular leaf spot and virus complex on pumpkin; bacterial spot on pepper; bacterial spot/speck, Fusarium wilt, early blight and blossom end rot on tomato.
On ornamentals, we have seen Pythium root rot on chrysanthemum; rust, Pythium root rot and take-all patch on turfgrass; cedar-quince rust on hawthorn; Verticillium wilt on maple; and anthracnose on yellowwood.

| UKREC-Princeton, KY, July 21 - 28, 2000 | |
| Fall Armyworm | 1 |
| Corn Earworm | 51 |
| European Corn Borer | 2 |
| Southwestern Corn Borer | 378 |
Dates and locations have been set for this fall's
commercial pesticide applicator training sessions for
Categories
1: Agricultural Plant
2a: Forest Pest
Control
3: Ornamental & Turf
4: Seed Treatment
10:
Demonstration & Research
12: Pesticide Dealer.
Visit the Pesticide Applicator Training website for details:
http://www.uky.edu/Agriculture/PAT/welcome.htm
Click on Pesticide Applicator Training Schedule.

Lee Townsend
Extension Entomologist