 Mark your calendar now for the 2002 IPM Training
School! Scheduled for March 20, the meeting will be
held at the UK Research Center in Princeton.
Registration will open at 8:30 AM with the meeting
starting at 9:00 AM and ending at 3:30 PM.
Mark your calendar now for the 2002 IPM Training
School! Scheduled for March 20, the meeting will be
held at the UK Research Center in Princeton.
Registration will open at 8:30 AM with the meeting
starting at 9:00 AM and ending at 3:30 PM.

 Mark your calendar now for the 2002 IPM Training
School! Scheduled for March 20, the meeting will be
held at the UK Research Center in Princeton.
Registration will open at 8:30 AM with the meeting
starting at 9:00 AM and ending at 3:30 PM.
Mark your calendar now for the 2002 IPM Training
School! Scheduled for March 20, the meeting will be
held at the UK Research Center in Princeton.
Registration will open at 8:30 AM with the meeting
starting at 9:00 AM and ending at 3:30 PM.
Pest identification will be a major part of the training school. Weed, insect and disease problems of corn, soybeans, small grains and alfalfa will be covered. An update of pest problems in Kentucky will include the following topics: Biology of Key Corn Diseases, Insect Damage in High Oil Corn and The Soybean Aphid in Kentucky. Also, the new computer program being used by the University of Kentucky Soil Testing Laboratory will be demonstrated.
Advance registration is not needed and the meeting is open to the public free of charge. The program has been accredited for 5.5 CEU's for Certified Crop Advisors. For additional information contact Patty Lucas at (270) 365-7541 extension 218 or plucas@uky.edu.
Look for Kentucky Pest News each week as the
growing season approaches. However, it will not be
sent out by First Class Mail. You can access it, and
previous issues, here on line. If you need a hard copy sooner,
you can print a PDF version from the link on the cover page.
You also can sign up
for automatic delivery via email by following
instructions on the cover page.

 Corn in which certain molds have grown can
sometimes be contaminated by mycotoxins (toxins
produced by fungi). Studies show that fumonisins are
the most frequent type of mycotoxin in corn in our
region and in the U.S. Fumonisins (fumonisin B1 and
related fumonisins) cause luecoencephalomalacia in
horses (ELEM, also called blind staggers and moldy
corn disease) and pulmonary edema in swine. Studies
also have raised concerns about possible cancer-
promoting activities of fumonisins in humans.
Because of these concerns, the U.S. Food and Drug
Administration last November published its
recommendations for maximum levels of fumonisins
in corn and corn by-products.
Corn in which certain molds have grown can
sometimes be contaminated by mycotoxins (toxins
produced by fungi). Studies show that fumonisins are
the most frequent type of mycotoxin in corn in our
region and in the U.S. Fumonisins (fumonisin B1 and
related fumonisins) cause luecoencephalomalacia in
horses (ELEM, also called blind staggers and moldy
corn disease) and pulmonary edema in swine. Studies
also have raised concerns about possible cancer-
promoting activities of fumonisins in humans.
Because of these concerns, the U.S. Food and Drug
Administration last November published its
recommendations for maximum levels of fumonisins
in corn and corn by-products.
The fungi that cause Fusarium kernel rot and produce fumonisins are called Fusarium verticillioides and Fusarium proliferatum. F. verticillioides is widespread in the midwestern and southeastern U.S., and the biology of this fungus is relatively well-understood. Kernels may become infected by F. verticillioides in several ways: via the silk channel; via systemic infection through the pedicel; and/or via the feeding activities of European corn borers. The role of the European corn borer is particularly important relative to this article for two reasons: (1) the feeding activities of this insect pest can both spread spores from plant to plant, and (2) the wounds created in kernels by feeding (by this or other insect pests) can allow kernel infection and high levels of fumonisin contamination in the kernels. For more information on the biology and symptoms of Fusarium ear rot, see the Extension Publication ID-121, "Mycotoxins in Corn Produced by Fusarium Fungi".
It is known that high levels of ear-feeding insects can enhance fumonisin levels in corn. Therefore, controlling insects that damage kernels can help reduce fumonisin contamination. Researchers at Iowa State University have shown that, under conditions of high pressure from the European corn borer, the use of Bt corn hybrids that express the endotoxin in the kernels themselves (MON810 and BT11 events) substantially reduced levels of Fusarium ear rot as well as levels of fumonisins. There was no consistent reduction in Fusarium ear rot or fumonisins for Bt hybrids with event 176, which expresses the endotoxin in green tissues and in pollen but not in kernels. In the Iowa studies, event DBT418 did not consistently reduce fumonisin levels either. Although event DBT418 is expressed in the kernels, the researchers attributed the lack of a benefit to generally poor late-season corn borer control with that event.
Significance for Kentucky Corn Producers
The Bt endotoxin is very active against both the
European corn borer and southwestern corn borer,
which both can create wounds on kernels that
potentially allow F. verticillioides to invade. If a
producer expects high levels of activity from corn
borers either European corn borer or Southwestern
corn borer use of a corn hybrid that expresses high
levels of the Bt endotoxin in kernels and provides
good late-season corn borer control (the MON810 and
BT11 events, for example) may result in lower levels
of fumonisin contamination in the harvested grain.
As a guideline, the greatest threat from these borers is
in late-planted corn. Use of Bt corn does not assure
the producer freedom from kernel-feeding insects,
since the Bt endotoxin only provides suppression
against the corn earworm and it provides no control at
all of fall armyworm. However, use of Bt corn
hybrids in fields where high insect pressure is
expected may reduce fumonisin contamination in
some cases. Understand that the use of Bt corn that
expresses the endotoxin in green tissues and pollen
only (event 176, for example) would not be expected
to consistently reduce fumonisin levels.
![]()
Soybean sudden death syndrome (SDS), caused by the soil-borne fungus Fusarium solani f.sp. glycines (FSG), has been known to occur in Kentucky since 1984. Epidemic years have been sporadic. However, serious outbreaks throughout west Kentucky during 2000, and the Green River Area during 2001, have gotten the attention of many producers.
Again, within the context of this article series, I am addressing the possibility that producers may be capable of doing a better job in managing SDS on the farm.
One of the first questions that usually comes up is the prospect of reducing the SDS potential of a field through crop rotation. Actually, crop rotation has not been found to reduce SDS or lower populations of the SDS causal fungus. FSG is a very common soil fungus and has a great capacity to survive in soil apart from a host plant. It is also very "happy" surviving on organic matter. None of these characteristics lend themselves well to management through crop rotation. There is one exception to this trend. Soybean cyst nematode (SCN) can be favorably impacted by crop rotations involving non-soybean crops. SCN is known to predispose soybean to early SDS symptom expression and more severe symptom development. Thus, it is logical that if one manages SCN through crop rotation, there should also be a concomitant reduction in the impact of SDS. This concept has not been examined sufficiently in field studies, but greenhouse studies clearly show SDS symptom expression is affected by SCN.
SDS is primarily a root rot disease. Foliar symptoms result from activity of one or more plant toxins produced by FSG in diseased roots. Plant roots are infected by the causal fungus early in the season, during the early vegetative period. Infection is favored in cool, wet soils. Consequently, any situation or practice that results in cool, wet soil, particularly early in the season, tends to encourage SDS. The most common situations that favor cool, wet soils are: 1) soil compaction (often associated with "heavy" soil types), 2) early planting dates, and 3) full-season no- till. There is not a great deal one can do to ameliorate wet-natured soils except tiling and divergence of surface water. However, if either or both these is feasible, the SDS situation in the field may be markedly improved. A great deal can be done to avoid early planting dates; however, there are often logistical considerations that must take priority. Nonetheless, the area of Kentucky that tends to have the most consistent and severe SDS problems (Green River Area) is also the area where very early planting dates (late April - very early May) are common. It may be appropriate to hold off planting until mid-May, especially in fields with a history of SDS.
Most no-till soybean fields in Kentucky are double- crop beans which are planted in late June to early July. These fields rarely show much SDS, probably due to the fact that warm soils, with moderate soil moisture, favor soybean root growth, but not the SDS pathogen. Full-season no-till beans are another matter. In a full- season environment, soil temps will remain low and soil moisture will remain high for a couple of weeks beyond what we see in a tilled crop. Thus, I would anticipate that no-till fields planted during the first two weeks in May would be at additional risk for SDS compared to a tilled crop planted during the same period. Some tillage when planting very early, or delayed planting when planting using no-till methods, may be options to consider in fields where SDS has been a problem.
Perhaps the most consistent and effective management of SDS is achieved by planting soybean varieties that have at least some SDS resistance. At present, a high level of SDS resistance does not exist in any variety. However, soybean varieties with moderate levels of SDS resistance can be readily found in maturity groups III, IV and V. The potential for SDS to reduce yields in a moderately resistant variety is much reduced compared to a SDS-susceptible variety. Some, but not all, SDS-resistant varieties are also resistant to SCN. Thus, if a soil test indicates that SCN is above threshold in a field that also has a history of SDS, it should not be too difficult to find a variety that will address both disease situations.
The impact of SDS in a crop is related to the level of crop health. Specifically, SDS tends to have less of a yield impact in vigorous, healthy crops than it does in stressed crops. Thus, some SDS benefit may be realized by doing as much as possible to encourage overall plant health. This includes effective management of other pests, maintenance of plant fertility, etc. Ironically, it is the fields with the greatest yield potential that often have the most serious problems with SDS. This is probably due to organic matter/soil quality characteristics that encourage both soil moisture retention and high soil fertility (and yield) and also survival and infection of roots by the SDS fungus. More aggressive producers that push planting dates also tend to farm fields with high yield potentials; thus, there may be sociological SDS management aspects that can be best addressed rural sociologists.
Historically, late-maturing varieties have been the hardest hit by SDS. This may simply be due to the fact that later maturing varieties are in the field longer than earlier varieties, which escape SDS. This association does not always hold up and I have seen some fields of early varieties severely damaged by SDS. Nonetheless, it may be wise select and plant early-maturing varieties in fields with a history of SDS; on average their SDS performance should be superior to late-maturing varieties (i.e., late group IVs - mid-Vs, depending upon location).
Finally, SDS can have indirect effects on crop yield. The most common indirect effects are shattering and poor seed quality associated with elevated levels of pod and stem blight. As plants with SDS are prematurely killed, any pods with grain are, in effect, ready for harvest. However, plants unaffected by SDS may not be ready for harvest. Farmers must, therefore, wait until the whole field is ready to begin harvest operations. The net effect is that the plants prematurely killed by SDS are subject to delayed harvest. Two common consequences of delayed harvest are shattering and elevated pod and stem blight. There is not a great deal a farmer can do to address this situation except to schedule fields that have SDS first for harvest and begin harvest operations as soon as possible.
Next in this series of articles, I will address fungal root and lower stem diseases.


 Degreeday accumulations are creeping up and signal
the approach of alfalfa weevil season in Kentucky. A
total of 190 dd (base 48) is the earliest time to begin
looking for weevil feeding in plant tips. As of March
11, totals for Bowling Green, Princeton, and Lexington
were 188, 215, and 131, respectively. An early check is
advisable for fields that had a problem with weevils
this year.
Degreeday accumulations are creeping up and signal
the approach of alfalfa weevil season in Kentucky. A
total of 190 dd (base 48) is the earliest time to begin
looking for weevil feeding in plant tips. As of March
11, totals for Bowling Green, Princeton, and Lexington
were 188, 215, and 131, respectively. An early check is
advisable for fields that had a problem with weevils
this year.
Very early tip feeding in alfalfa usually is caused by the clover leaf weevil (CLW) but resembles that of the alfalfa weevil (AW). It is a green, legless grub that looks very similar to the AW larva but can be recognized by looking closely. The CLW larva has a light brown head compared to the dark brown head of the AW. A white stripe that runs down the middle of the back of the CLW has a thin pink border on each side. The stripe on the AW does not. Finally, there is a distinct behavioral difference. CLW larvae feed at night and are on the ground around the base of the plant during the day. AW larvae remain in the tips at all times.
CLW overwinter as partly grown larvae and complete their development early in the spring. Usually, they are not abundant enough in clover or alfalfa to justify control because a fungal disease provides excellent natural control. They may reach damaging levels during unusually dry springs.
See Insect Recommendations for more alfalfa pest recommendations.

Ticks prefer to live in woods, tall grass, weeds and brush. They climb onto low vegetation and attach to suitable hosts which pass by, including pets and people.
Ticks tend to be less of a problem well-maintained lawns although edges of property supporting tall weeds and brush can be a source of infestation. The best way to avoid acquiring ticks is through prevention:
1. Avoid walking through uncut fields, brush and other areas likely to harbor ticks. When hiking or picnicing in these areas, wear long pants tucked into socks and consider using tick repellents. Walk in the center of mowed trails to avoid brushing up against vegetation.
2. Inspect family and pets after being in tick-infested areas, and promptly remove any ticks which are found (ticks most often attach at the neck and scalp).
3. Keep grass and shrubs in your yard trimmed, and clear overgrown vegetation from edges of your property. Ticks avoid direct sunlight and will not infest areas which are well maintained.
4. Free-roaming pets are much more likely to become infested with ticks than are those which are confined. Pets may be treated with insecticide dips or sprays, although these products generally lose effectiveness in about a week.
5. Treating lawns is of little benefit since this is not a preferred habitat for ticks. If insecticides are used, treatment should be concentrated in areas where pets, rodents, and other potential wild hosts of ticks are likely to frequent, e.g., dog house, fenceline, and along margins between wooded or brushy areas and the lawn. Sevin (carbaryl), Bayer Advanced Lawn and Garden Multi-Insect Killer (cyfluthrin) and Ortho Bug B Gon (permethrin) are examples of effective materials. Check insecticide labels for products that contain these active ingredients.
Remove attached ticks carefully using a fine-point tweezers. Grasp the tick just behind the point of attachment and pull slowly and steadily until the tick is dislodged. Vaseline, matches and other alternate methods of removal should not be used. Wash the bite area, apply antiseptic and cover with a band-aid.
 This is the time of year when home gardeners are
"digging in" and "pruning out" around the yard and
garden. Gardeners working with woody plants often
prune out diseased plant parts to improve their
plants' health. Spading the garden helps to bury old
plant material and create beds for crop rotation.
However, there is often a concern about whether or
not one needs to clean up and sanitize garden tools
before using them in trees and flower beds. Sanitizing
yard and garden tools is an important part of garden
disease management. The following guidelines may
help home gardeners in deciding to what extent tools
need to be sanitized.
This is the time of year when home gardeners are
"digging in" and "pruning out" around the yard and
garden. Gardeners working with woody plants often
prune out diseased plant parts to improve their
plants' health. Spading the garden helps to bury old
plant material and create beds for crop rotation.
However, there is often a concern about whether or
not one needs to clean up and sanitize garden tools
before using them in trees and flower beds. Sanitizing
yard and garden tools is an important part of garden
disease management. The following guidelines may
help home gardeners in deciding to what extent tools
need to be sanitized.
Outdoor tillage tools: Tillage tools used out-of-doors in flower and vegetable beds may include spades, power tillers, rakes, hoes and trowels. Dirty tillage tools can carry microbes causing plant diseases including root-knot nematode, black root rot, root and collar rot, and Verticillium wilt. When gardeners bring in a power tiller or spade that has been used in another garden, or reintroduce tools used in the garden last year, they should be sanitized before use. Previously used and dirty tools should be scrubbed with a brush and soap and water and rinsed and dried before using them in the garden.
For tools used in the greenhouse or transplant beds: These include trowels, dibbles, pots and flats used in growing transplants or other tender plants. Here, one risks introduction of common soil pathogens such as Rhizoctonia, Pythium, and Phytophthora into pots, flats, etc. containing pathogen-free soil mixes where the introduced microbes can run rampant. For these situations, first wash, rinse, and dry the tools. Then, dip them in 10% bleach, or in 70% alcohol, or in Lysol and rinse them in clean water. Each dipping period should last 15-30 seconds. Be aware that bleach can be corrosive (hence the need to rinse the tools) and that care should be taken to avoid breathing the fumes.
Pruning tools: During the dormant season, loppers, clippers and saws are used for cutting diseased branches and stems of woody plants. Washing, rinsing and drying should prevent spread of major diseases such as Verticillium wilt, fire blight, Dutch elm disease, bacterial leaf scorch, and canker diseases during dormant pruning.
For pruning tools being used during the growing
season on already-diseased plants (to avoid disease
spread to other plants nearby): Prune diseased plants
last. Then, when pruning plants with fire blight,
Verticillium wilt, Dutch elm disease, chestnut blight,
bacterial leaf scorch, rose viruses, crown gall, etc.,
prepare to sanitize pruning tools before moving on to
the next cuts. Pruning tools can be dipped in 10%
bleach, 70% alcohol, or Lysol and rinsed as described
above. Also, as previously noted, for dormant
pruning, sanitizing tools between cuts is not normally
needed.

 Several brightly colored beetles have been sent in from
houses over the past few days. Most common is a 5/8
inch long black beetle with yellow lines across the
segment behind the head and thin yellow lines and
"w"-shaped markings on the wing covers, the painted hickory borer.
A pair of long antennae reach half way
back along the body.
Several brightly colored beetles have been sent in from
houses over the past few days. Most common is a 5/8
inch long black beetle with yellow lines across the
segment behind the head and thin yellow lines and
"w"-shaped markings on the wing covers, the painted hickory borer.
A pair of long antennae reach half way
back along the body.
A variety beetles and other insects can emerge from firewood that has been indoors during the winter. Fortunately, they do not infest structural wood in a home or building. They can be released outdoors or swatted, depending on your frame of mind at the time.
A quick inspection of firewood probably will reveal some exit holes in the wood and piles of sawdust. More information on firewood insects is available in ENTFACT 626.
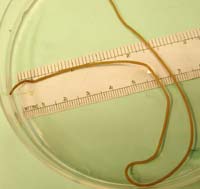 Horsehair worms, also known as Gordian worms, are
similar to nematodes but are about 4 inches long and
about 1/10 inch in diameter. They usually are found
in water or wet areas such as cisterns, livestock
watering troughs, streams, puddles, and even on
plant foliage.
Horsehair worms, also known as Gordian worms, are
similar to nematodes but are about 4 inches long and
about 1/10 inch in diameter. They usually are found
in water or wet areas such as cisterns, livestock
watering troughs, streams, puddles, and even on
plant foliage.
They are not parasites of humans, livestock, or pets. However, the immature stages (larvae) do develop in grasshoppers, crickets, and some beetles and cockroaches. The mere sight of these curious, writhing worms can cause quite a stir.
Adults mate in water and females lay long gelatinous strings of eggs. A mass can contain several million eggs. Depending on water temperature, the eggs hatch in two weeks to three months. The life of the microscopic larvae is not completely understood. Within 24 hours after hatching, they are thought to form a protective covering or cyst on vegetation near the water's edge. If the cyst is eaten by a suitable insect, the protective covering dissolves. The released larva bores through the gut wall and into the body cavity of the host. There, it digests and absorbs the surrounding tissue. Mature worms leave the host to find a mate.
These long, slender creatures are harmless to humans,
pets, and livestock. There is no need for control
measures. Their presence in cisterns indicates there is
some way that crickets or other host insects can get in
or that groundwater is entering the system. Check for
cracks or openings that can be screened or sealed. The
horsehair worms are not a problem but contamination
from other sources can be.
The Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) allows individual states some latitude to implement "Special Local Need" (SLN) registrations of pesticide uses important and unique to a particular state. Although there are instances of 24(c) registered uses approved independently in several states, the label is still regarded as a state label and is regulated as such. The part of FIFRA that describes this authority is Section 24(c); consequently these registrations are often referred to as 24(c) registrations.
The University of Kentucky assists in the registration process by reviewing the support material submitted by the registrant to determine if certain criteria are satisfied. Once this review is completed, a recommendation is made to the Division of Pesticides regarding whether to approve or deny the registration. Since the Division of Pesticides is the state lead agency for regulating pesticides, it has the responsibility and authority on approving or denying 24(c) registrations. Once a 24(c) has been approved by the Division of Pesticides, it is reviewed by the Federal EPA, which has the general oversight of 24(c) registrations.
Based on the current policy of the Division of Pesticides, 24(c) registrations expire on December 31 of the third year from which they are approved. For example, if a registration is approved on August 21, 2002, it will expire December 31, 2004. SLN registrations are eligible for renewal if reviewed and approved according to the state's guidelines.
Listed below are 24(c) registrations approved specifically for Kentucky.
| Product Name | SLN Registration Number | Expiration Date |
|---|---|---|
| Command 4EC (For use on winter & summer squash) | KY-93002 | 12/31/2002 |
| Dithane DF (For use on tobacco seedlings grown for transplanting) | KY-940002 | 12/31/2002 |
| Command 4EC (For use on direct seeded and transplanted cabbage) | KY-960003 | 12/31/2002 |
| Poast Herbicide (For use on tobacco) | KY-970002 | 12/31/2002 |
| Dual Magnum (For use on transplanted bell peppers) | KY-990001 | 12/31/2002 |
| Dual Magnum (For use on transplanted cabbage) | KY-990002 | 12/31/2002 |
| Acrobat MZ (For controlling metalaxyl/mefanoxam-resistant blue mold in field-grown tobacco) | KY-990003 | 12/31/2002 |
| Terramaster 35 WP (For pythium root rot control in tobacco transplant float beds) | KY-000001 | 12/31/2002 |
| Tracer (For control of tobacco budworm and tobacco hornworm) | KY-000002 | 12/31/2002 |
| Ferbam Granflo (To control blue mold in tobacco seedlings) | KY-940001 | 12/31/2003 |
| Pounce 3.2 EC (For armyworm control in hayfields) | KY-010001 | 12/31/2003 |
Lee Townsend
Extension Entomologist