 Active blue mold has been reported from the following list of
counties. Please check the list and if the disease is active in your
county and we do not have your county in the list below, let the
local county extension office know.
Active blue mold has been reported from the following list of
counties. Please check the list and if the disease is active in your
county and we do not have your county in the list below, let the
local county extension office know.

 Active blue mold has been reported from the following list of
counties. Please check the list and if the disease is active in your
county and we do not have your county in the list below, let the
local county extension office know.
Active blue mold has been reported from the following list of
counties. Please check the list and if the disease is active in your
county and we do not have your county in the list below, let the
local county extension office know.
As of press time on June 28, counties with one or more confirmed cases of blue mold included: in Kentucky - Adair, Anderson, Bath, Bourbon, Boyle, Bracken, Casey, Clark, Cumberland, Fayette, Fleming, Jessamine, Garrard, Green, Harrison, Hart, Henry, LaRue, Lawrence, Lewis, Lincoln, Logan, Madison, Mason, Menifee, Mercer, Morgan, Montgomery, Nicholas, Pulaski, Robertson, Rowan, Scott, Taylor, Warren, Washington, and Woodford counties; in Tennessee - Union, Macon, Robertson, and Smith counties; western North Carolina - Madison County; southern Ohio - Adams, Brown, Highland and Scioto counties; and western Virginia - Lee County.
Blue mold continues to increase in Kentucky, concentrated along the general southwest to northeast corridor mentioned in the previous reports, that runs in the southwest roughly from Springfield, TN , northeast through Kentucky into southern Ohio. See the Regional Status maps at http://www.uky.edu/Agriculture/kpn/kyblue/kyblu04/statmap.htm. A blue mold advisory exists for the entire burley production area, with watches for the eastern two- thirds of Kentucky, southern Ohio, and western West Virginia. Warnings exist for all counties with confirmed cases of blue mold.
The crop development stages in Kentucky range widely, from yet to be transplanted through just topped. As growers actually go into their fields to cultivate and make insecticide applications, many are finding more blue mold than they had expected. However, in other cases, the disease is not found in fields which are often the first to develop it in a community. Activity is increasing somewhat all along the corridor, but most new activity is being reported north and east in and near this corridor.
Additionally, rumors (but without confirmation) are common of new activity in western Kentucky along a path from Cross Plains, Tn. to the Ohio River near Owensboro, Ky. Our labs have diagnosed spotting related to herbicides (lesions somewhat resembled blue mold, but no pathogen was found in them) in some of the suspect cases in this region, however. This is why it is important to have cases confirmed by pathologists. Frogeye leaf spot, which also produces a yellow lesion, is common in some fields and it is being confused with blue mold, especially where the lesion is accompanied by heavy sporulation. Sporulation on the frogeye lesion will be in the dead area of the lesion, only, and mainly on the top side of the lesion. The blue mold pathogens makes spores in live tissue and mainly on the lower side; so always check the live areas of the lesion for spores as any blue mold sporulation in the dead areas is old sporulation.
County Extension Agent reports last week indicated that blue mold was still strongly associated with setting infected/infested transplants, but by late in the week, several were indicating that strong blue mold was now occurring in the early set tobacco - some fields with widespread development of economically damaging levels on the first strike. Systemic activity is very common in some communities, mainly due to setting infected transplants.
Several dealers have reported, and Syngenta representatives have confirmed, that supplies of Actigard 50 W (the plant inducer) are limited in the burley region. Efforts are underway to locate and redistribute some stocks allocated to other areas.
For the latest blue mold status and other tobacco disease information, check the KY Blue Mold Warning System online.
![]() http://www.uky.edu/Agriculture/kpn/kyblue/kyblue.htm
http://www.uky.edu/Agriculture/kpn/kyblue/kyblue.htm

For more information about tobacco pests, visit "Insect Management Recommendations".

 As the summer months roll on, corn growers in the
western part of the state are reporting large numbers of
Japanese beetles attacking corn just as is it beginning to
silk. Over the past decade, we have seen these excessive
Japanese beetle infestations move across the state from east
to west. During the first 4 or 5 years that Japanese beetle
invades into an area, extreme numbers of adults can attack
various preferred hosts. We are finding high populations
in corn in western Kentucky, particularly west of a line
extending from Hopkinsville to Henderson.
As the summer months roll on, corn growers in the
western part of the state are reporting large numbers of
Japanese beetles attacking corn just as is it beginning to
silk. Over the past decade, we have seen these excessive
Japanese beetle infestations move across the state from east
to west. During the first 4 or 5 years that Japanese beetle
invades into an area, extreme numbers of adults can attack
various preferred hosts. We are finding high populations
in corn in western Kentucky, particularly west of a line
extending from Hopkinsville to Henderson.
While Japanese beetles attack over three hundred species of plants, in agricultural areas, they will attack corn and soybeans in large numbers. In corn, the preferred site of attack is the ear silk. When numbers are high, twenty or more beetles may be found on the tip of a single ear. The beetles clip the silks back into the tip of the ear. If the beetles clip the silks before the corn has a chance to pollinate, then the beetles can prevent pollination to some or all of the ears. This can results in a barren cob.
The key to managing Japanese beetles feeding on corn silks is determining if pollination has yet occurred. If the plant has already shed pollen and the ear has pollinated, then the beetles only cause little damage to the corn. Generally, pollen shed occurs one or two days before the ears become receptive to the pollen. If the silks have been clipped prior to pollination, they will continue to grow. So if pollination has not occurred, and two or more beetles are attacking each ear and keeping the silks at 1/2 inch or less, then the beetles may need to be controlled.
As growers visit fields to evaluate Japanese beetle infestations, they need to keep in mind that the outside 10 to 20 rows usually have much higher numbers than the interior of the field. This is because most of the beetles colonize from outside of the field. Our experience has shown us that growers can often get by with treating just the outside rows rather than the entire field. While infestations will last several weeks, it is only during the critical time for pollination that growers need to manage these beetles in corn.
There are several insecticides that effectively control Japanese beetles in corn, but due to the nature of corn silk growth, only limited residual activity can be expected.
For information about corn pests, visit
"Insect Management Recommendations".


![]() Last season there was considerable interest in spraying
soybean in the early pod stages with a combination of
Quadris (6.2 fl oz /A) + Warrior (2.56 fl oz/A). Based on
our discussions with farmers, consultants and
agrichemical dealers, we estimate that about 30,000 acres
were sprayed in 2003. Our contacts also indicate that there
is even more interest this year in light of the strong
soybean market and word-of-mouth "advertising" by
many farmers who experienced good results with the
treatment in 2003.
Last season there was considerable interest in spraying
soybean in the early pod stages with a combination of
Quadris (6.2 fl oz /A) + Warrior (2.56 fl oz/A). Based on
our discussions with farmers, consultants and
agrichemical dealers, we estimate that about 30,000 acres
were sprayed in 2003. Our contacts also indicate that there
is even more interest this year in light of the strong
soybean market and word-of-mouth "advertising" by
many farmers who experienced good results with the
treatment in 2003.
In early February of this year we wrote a fairly comprehensive Kentucky Pest News article (http://www.uky.edu/Agriculture/kpn/kpn_04/pi04020 9.htm ) on the Quadris + Warrior treatment. Not much has changed since that article was published. Two things were very obvious by the end of last season. First, the Quadris + Warrior treatment does show considerable promise, even though we have yet to account for the apparent yield impact (commonly in 3-8 bu/A range) by the treatment. By way of reminder, the results of two replicated field experiments in 2003 indicated that only the combination treatment (Quadris, 6.2 fl oz/A + Warrior, 2.56 fl oz/A), but neither treatment applied alone, significantly increased yields. However, in those same research plots, Quadris had the same impact as Quadris + Warrior in terms of disease control (stem anthracnose was controlled), and delayed maturity. Warrior, on the other hand, did not affect maturity and insect pests did not appear to be at yield-limiting levels. Yet, it took mixing Warrior with Quadris before yield increases were evident!
The second obvious finding from last year is that applying Quadris + Warrior does NOT always result in increased yields. Yes, some producers were very pleased with the yield results of the treatment last season. Others, however, were very disappointed by the apparent lack of a yield response to the treatment. Clearly applying Quadris + Warrior does not assure a yield increase, and anyone making the application this season should consider no yield response as a definite possibility. Nonetheless, based upon our research and a summary of yield data from many fields last year, it does appear that the odds favor yield increases, at least based on 2003 results.
Our research, as well as the experience of numerous farmers last year, suggests that late-planted crops and late maturing varieties may be the least likely to show an economic yield response to Quadris + Warrior. Our goal for this season is find out why applying Quadris + Warrior substantially increases yields in some situations, yet does not have a positive yield outcome in others. We watched and scouted large and small plots last year and nothing, pest wise, really floated to the surface except stem anthracnose. At harvest, stem anthracnose was very evident in almost every field we looked at, and the Quadris + Warrior treatment reduced this disease by 40- 50% in most cases. But as discussed above, Quadris alone also reduced stem anthracnose, but with no yield response. So whatever is going on is not very obvious.
This season we have planted four experiments at the UKREC in Princeton aimed at studying various factors that may be involved in this complex situation (planting date, variety, timing of application, chemical). By the end of the season we hope to have a much clearer understanding of the yield / Quadris + Warrior interaction.
![]() Most of you are aware of the soybean rust threat to the
United States soybean crop. Fortunately, as of this writing,
soybean rust has yet to be found in the continental U.S.
Once soybean rust is found here, the only practical control,
at least in the short term, will be to apply foliar fungicides.
One major hurdle we have to overcome, is that the only
fungicide labeled for use on soybean in the U.S., that is
also highly effective against soybean rust, is azoxystrobin
or Quadris, a Syngentia product. Everyone is in agreement
that the supplies of Quadris would be depleted rapidly if
no other effective products were available for farmers to
use. Keep in mind that there are 73 million acres of
soybean in the U.S.!
Most of you are aware of the soybean rust threat to the
United States soybean crop. Fortunately, as of this writing,
soybean rust has yet to be found in the continental U.S.
Once soybean rust is found here, the only practical control,
at least in the short term, will be to apply foliar fungicides.
One major hurdle we have to overcome, is that the only
fungicide labeled for use on soybean in the U.S., that is
also highly effective against soybean rust, is azoxystrobin
or Quadris, a Syngentia product. Everyone is in agreement
that the supplies of Quadris would be depleted rapidly if
no other effective products were available for farmers to
use. Keep in mind that there are 73 million acres of
soybean in the U.S.!
With the above situation in mind, a group of scientists, with Minnesota and South Dakota taking the lead, developed a "national section 18 application" and submitted it to the Environmental Protection Agency (EPA). The application requested approval for use of multiple fungicides (primarily triazoles) once soybean rust is found in the U.S.. States were given the option to "sign on" to the section 18 application, which Kentucky did. As of now, EPA has approved the use of mycolbutanil (Laredo EC and Laredo EW [Dow Agrosciences]) and propiconazole (Bumper [Makhteshim-Agan], Propimax [Dow], and Tilt [Syngentia]) in Kentucky should soybean rust be found in the U.S. and require control.
Note: the section 18 DOES NOT GO INTO EFFECT until soybean rust is confirmed in the United States. Until then, it is ILLEGAL to use any of the aforementioned section 18 fungicides on soybean in Kentucky.
I expect EPA to approve additional fungicides in the next couple of months. I will keep you informed each time another section 18 fungicide is approved. However, I did think it was important to inform you now that proactive steps are being taken in preparation for the eventual arrival of soybean rust in Kentucky.
For more information about soybean pests, visit
"Insect Management Recommendations".


We have diagnosed a number of cases of Pythium root rot on new seedings of alfalfa this spring, particularly those that were seeded in late April and early May, just before the onset of a sustained period of soggy weather. Commonly these plants are exhibiting stunting, yellowing, reddening, and defoliation. These symptoms are the result of the damaged root system, which can be observed if plants are carefully dug and root systems washed.
The Pythium organisms that attack alfalfa and seedlings of many other crops are very common in out soils. It is probably impossible to find an agricultural soil in Kentucky that is completely free of infectious Pythium. The frequent, heavy rains that fell since early May provided substantial opportunity for these organisms to be active and infect unprotected alfalfa seeds and seedlings. These are the very weather conditions that justify the routine use of a seed treatment to protect against Pythium.
All alfalfa varieties should be pre-treated before sowing with either metalaxyl or mefanoxam (also called metalaxyl-M). Example products include Allegiance, Apron, and others. This seed treatment will provide roughly two weeks of protection against Pythium infections. While this may not be enough in the most extreme conditions of wet conditions, it goes a long way to helping get the stand established in a wet spring.
High-yielding, multi-pest resistant alfalfa varieties often are treated with either of these active ingredients. However, many of the "low-budget" alfalfa varieties on the market ("Buffalo", etc) are not, and these are the ones we've been seeing in the Diagnostic Labs.
Based on recent industry figures, it costs about 5¢ per pound of seed to treat with these fungicides, which is no more than about a dollar per acre sown. Everyone who knows me knows how thrifty I am, so I can identify with the desire to save costs. However, these seed treatments are like insurance, and inexpensive insurance at that. Many times, alfalfa will be successfully sown without it. However, every now and then, when conditions are right, it will be the difference between successful establishment or failure.
The need for such seed treatment is much lower in late- summer seedings, because of the generally lower rainfall and faster drying conditions of the soil at that time.
Bottom line: Consider treating all alfalfa seed sown in the spring with metalaxyl or mefanoxam (also called metalaxyl-M).
See Insect Recommendations
for more alfalfa pest recommendations.


 "My apple tree leaves have rust colored spots on them;
what is it and what can I do about it?" This is a common
home garden question being asked this year. The short
answer is that growers are seeing cedar-apple rust on their
apple trees. But there are other kinds of cedar rusts of
apple; and, other plants such as crabapple and hawthorn
are also affected. Cedar rusts are widespread this year
because of the wet spring weather favored disease
development.
"My apple tree leaves have rust colored spots on them;
what is it and what can I do about it?" This is a common
home garden question being asked this year. The short
answer is that growers are seeing cedar-apple rust on their
apple trees. But there are other kinds of cedar rusts of
apple; and, other plants such as crabapple and hawthorn
are also affected. Cedar rusts are widespread this year
because of the wet spring weather favored disease
development.
Three related rust diseases occur on apple trees in Kentucky: cedar-apple rust, cedar-hawthorn rust, and cedar-quince rust. Crabapple, hawthorn, mountain ash, pear, and serviceberry are also susceptible to these diseases. All three rusts are caused by different species of the fungus Gymnosporangium, each of which must spend a phase of its life cycle as a parasite on Juniperus species such as native red cedars or ornamental junipers.
Although cedar rusts can cause unsightly growths on Juniperus, they do not usually cause serious damage to these plants. However rust diseases can cause serious fruit losses on apples and weaken and kill shoots of crabapples and hawthorns. Infected fruits can drop prematurely or have a reduced commercial value if they remain on the tree through harvest. Leaf infections often result in premature leaf loss, reducing the size and quality of the fruit crop, weakening the tree, and reducing bloom the following year. Hawthorn and crabapple twigs infected by the cedar-quince fungus can become swollen and die.
Symptoms. Cedar-apple rust, caused by G. juniperi- virginianae is the most common and important of the apple and crabapple rust diseases on apple. On apple leaves, small, pale yellow spots (sites of rust fungus infection) appear on the upper surface in mid to late spring. Spots gradually enlarge (up to ¼-inch in diameter, depending on the apple variety and the number of spots per leaf) and become bright yellow-orange frequently surrounded by a reddish border. As the spots enlarge, black dots (a mass of fungal fruiting bodies called pycnia) develop in the centers. Shortly thereafter, the fungus grows through to the lower leaf surface, where yellow spots also appear, and the tissue becomes noticeably thickened. In late spring or early summer, clusters of small orange-yellow, tubular fruiting bodies (aecia) project downward from these lower surface spots. This is the stage that is being observed by growers now. As the "tubes" mature, they split toward the base in narrow strips and curl back on themselves to form "cups" within which a mass of light brown spores is revealed. Infected leaves may turn yellow and drop, especially as the tree becomes stressed for water.
Cedar-hawthorn rust, caused by G. globosum, causes leaf spots of apple, crabapple, hawthorn, pear, and serviceberry which are similar in appearance, but few tubular aecia form within them. Cedar-quince rust, caused by G. clavipes, does not cause leaf spots on these hosts.
Cedar-apple rust-infected apple fruit develop spots appearing near the blossom end. They are yellow-orange in color, like the leaf spots, but are much larger (up to ¾- inch or more in diameter), and are surrounded by a dark green zone on the otherwise light green fruit. The tubular aecia frequently fail to develop but when present, they are usually found in a circle surrounding the black dots (pycnia) which form on a raised, roughened cushion of tissue. Tissue below the spots turns somewhat corky, but remains alive. Infected fruits frequently become deformed and may drop prematurely. Similarly, apple fruit infected by the cedar-quince rust fungus become puckered at the blossom end while still an inch or less in diameter, then develop sunken, dark green spots. In contrast with cedar- apple rust, the flesh below cedar-quince rust spots is dead, brown, and spongy, often all the way to the core. Pycnia and aecia rarely develop, making positive diagnosis difficult. Apple fruit infection by the cedar-hawthorn rust fungus is rare. The cedar-quince rust fungus commonly infects hawthorn fruits. Such fruits become abnormally swollen and show profuse numbers of the tubular aecia.
Cedar-apple rust normally does not affect apple twigs. Hawthorns and crabapple twigs infected by the cedar- quince fungus can become swollen and die.
Cedars (junipers) are alternate hosts for the cedar rusts, but obvious rust disease symptoms are not now evident on them. The cedar-apple rust fungus forms light brown to reddish or chocolate brown galls in the leaf axils of infected Juniperus species. These galls are usually rounded and range from pea-sized to 2 inches in diameter. As galls mature, the flesh becomes corky and the surface becomes pitted with circular depressions. In spring, following rainy periods, slimy, yellow-orange tendrils, or "spore horns," up to 2 inches long swell and protrude from these depressions. A gall may produce many spore horns, which cause it to resemble orange-colored blossoms from a distance. Severely rusted Juniperus can be very conspicuous.
Galls produced by cedar-hawthorn rust (G. globosum) are similar in appearance but are smaller and more irregular in shape and do not develop the regular arrangement of circular depressions. Spore horns, too, are shorter, generally fewer in number, and wedge- or club-shaped. Cedar-quince rust (G. clavipes) does not form rounded galls but instead forms perennial, spindle-shaped swellings on the twigs, on which a gelatinous, orange-brown mass of spores is borne in the spring.
Disease cycle. All three fungi have similar life cycles. Aeciospores (being produced now in the tubular aecia on diseased apple, crabapple, or hawthorn tissue) are blown to cedar during the summer where they germinate, infect, and cause small pea-sized galls to form on the twigs. The fungus over-winters in these galls which continue to grow and enlarge during the following year. The fungus survives a second winter within the gall, then begins producing its long, orange spore horns the next spring, about the time the apple buds are in the pink to early bloom stage. These spore horns are actually columns of fungal spores (teliospores), each of which can germinate under moist conditions to form four new spores (basidiospores). Basidiospores are then carried to apple trees by wind currents where they can germinate and cause infection during relatively short periods of wetness (about six or seven hours for moderate levels of infection when temperatures are in the 50s and 60s). The danger of infection usually ends about 30 days after bloom when the fungus terminates production of basidiospores on cedars and the majority of apple leaves have aged to the point where they are no longer susceptible. Spots begin forming on the upper leaf surface about 10 to 14 days after infection occurs, and aecia form on the underside of infected leaves several weeks later. Spores produced within the aecia are then blown to nearby cedars and junipers, completing the cycle that began two years earlier.
Cedar rust disease management.


 Small, elongate feeding holes and larger, dried, brown
areas of poplar leaves signal the activity of yellow poplar
weevils. The small black adult weevils chew the rice grain-
shaped holes in the leaves while the grub-like larvae mine
within the leaf tissue. This insect also feeds on sassafras
and magnolia. More information is available in Entfact 414
- "Yellow poplar weevil" which can be found at
http://www.uky.edu/Agriculture/Entomology/entfacts/trees/ef414.htm
Small, elongate feeding holes and larger, dried, brown
areas of poplar leaves signal the activity of yellow poplar
weevils. The small black adult weevils chew the rice grain-
shaped holes in the leaves while the grub-like larvae mine
within the leaf tissue. This insect also feeds on sassafras
and magnolia. More information is available in Entfact 414
- "Yellow poplar weevil" which can be found at
http://www.uky.edu/Agriculture/Entomology/entfacts/trees/ef414.htm


 Abundant rainfall has jump-started the mosquito season in
Kentucky. The risks of contracting West Nile Virus are
slight, and in most affected individuals, the symptoms and
health consequences are not severe. Nonetheless,
mosquitoes remain a perennial, warm-season pest for
which there is no easy solution. As summer continues,
there will be an abundance of misinformation about what
works and what does not. Countless products will claim
ease and effectiveness, but few have appreciable value in
lessening the annoyance and incidence of bites.
Unlike most insects encountered around homes,
mosquitoes are pervasive outdoor pests and there are
limits to what can be done to minimize their abundance.
The following measures, however, can afford some relief.
Abundant rainfall has jump-started the mosquito season in
Kentucky. The risks of contracting West Nile Virus are
slight, and in most affected individuals, the symptoms and
health consequences are not severe. Nonetheless,
mosquitoes remain a perennial, warm-season pest for
which there is no easy solution. As summer continues,
there will be an abundance of misinformation about what
works and what does not. Countless products will claim
ease and effectiveness, but few have appreciable value in
lessening the annoyance and incidence of bites.
Unlike most insects encountered around homes,
mosquitoes are pervasive outdoor pests and there are
limits to what can be done to minimize their abundance.
The following measures, however, can afford some relief.
Eliminate Breeding Sites - Mosquitoes need quiet, non- flowing water for their development. Eliminating sources of standing water, such as swamps and ditches, may require community-wide effort. Nonetheless, homeowners can take steps to prevent mosquitoes from breeding on their property:
Larval Control - Use of a mosquito larvicide may be beneficial when it is impractical to eliminate a breeding site. Larvicides are insecticides used to control immature mosquitoes before they have a chance to develop into biting adults. Most larvicides sold to homeowners contain either the ingredient methoprene, or the bacterium Bacillus thuringiensis israelensis (Bti). Neither active ingredient is harmful to fish, waterfowl, pets or humans when used according to label directions.
Many products and formulations containing methoprene (Altosid®) and Bti (Bactimos®, Vectobac®) are used by mosquito abatement agencies and other professionals. Homeowners can purchase the methoprene-based larvicide, PreStrike™ in hardware, discount, and some pet stores. PreStrike is formulated as a granule and comes in a shaker bottle. Various products containing the mosquito- specific bacterium, Bti, are also sold to homeowners. Mosquito Dunks® and Quick Kill® Mosquito Granules, for example, can be found at hardware and discount stores.
Adult Control - Adult mosquitoes prefer to rest in moist, quiet, shaded areas such as dense vegetation during the daytime. Consequently, homeowners should remove tall weeds and overgrown vegetation from their yards. To further reduce intolerable levels of biting adult mosquitoes, insecticides can be applied to shrubs, hedges and other shaded areas, such as under decks and along foundations. Lawn and garden insecticides containing pyrethroids (e.g., permethrin, Ortho Mosquito B Gone or Spectracide Mosquito Stop; cyfluthrin, Bayer Advanced Powerforce Mosquito Killer; lambda cyhalothrin, Spectracide Triazicide), are effective but will need to be periodically reapplied. A hose-end sprayer is usually most effective, and convenient formulations for making such applications are widely available. For such applications, some homeowners may wish to enlist the services of a professional pest control firm.
Exclusion - Mosquitoes can be kept out of homes by securely screening windows, doors and porches. The occasional mosquito found indoors can be eliminated with a fly swatter. Aerosol-type insecticides labeled for mosquitoes, gnats, and other flying insects seldom provide much relief at the dosages applied by householders.
Topically-Applied Repellents - Repellents will help prevent bites when spending time outdoors. The most effective mosquito repellents contain the active ingredient diethyl toluamide (DEET). Higher percentages of DEET in the ingredients provide longer protection. Low -percentage formulations (10% or less) are available for use with young children. Non-DEET containing repellents (e.g. Avon Skin- So-Soft7, citronella) may provide some relief, but to a lesser degree and for shorter duration than DEET- containing products. It is often desirable to apply insect repellent on outer clothing as well as the skin. Always read and follow directions on the container. Mosquito repellent should not be applied to the hands of young children, and treated skin should be washed with soap and water after returning indoors.
Other Control Possibilities - Many consumer products claim to attract, repel, capture or kill mosquitoes. Most of these devices do not appreciably reduce mosquito abundance or incidence of bites, or else their claims are unproven. Electrocuting devices or "bug zappers" using ultraviolet light as an attractant are generally ineffective in reducing outdoor populations of mosquitoes and their biting activity. Studies indicate that mosquitoes make up only a tiny percentage of the insects captured in such traps. The majority are moths, beetles and other harmless night flying insects.
Other types of mosquito traps utilize carbon dioxide, warmth, light, and various chemicals (e.g. octenol) as attractants and claim to capture tremendous numbers of adult mosquitoes. Such devices can be quite expensive. Performance claims to the contrary, such traps seldom have been shown to reduce populations of biting mosquitoes on one's property, or the frequency of bites. In some situations, they could even attract more mosquitoes into the area they were meant to protect.
Advertisements for portable electronic devices using high frequency, ultrasonic sound routinely appear in magazines, claiming to keep mosquitoes and other pests at bay. Some supposedly repel mosquitoes by mimicking the wing beat frequency of a hungry dragonfly. Scientific studies have repeatedly shown these devices to be of negligible benefit in deterring mosquitoes and reducing bites. Save your money, as these devices seldom if ever provide any appreciable measure of protection.
Citronella oil does have mosquito-repelling properties, and the scented candles can provide some protection. For maximum effect, use multiple candles placed close (within a few feet) of where people are sitting. A single candle located at the center or edge of a picnic blanket probably won't provide much benefit other than atmosphere. Mosquito-repellent plants, garlic, and other oft-advertised botanical products generally are ineffective.
Bats and certain types of birds (e.g. purple martins) are
often cited as effective natural agents for managing
outdoor mosquitoes. Conservation groups and nature
magazines often suggest building bat and birdhouses on
one's property to promote nesting and to protect against
mosquitoes. Although insectivorous bats and birds do eat
mosquitoes, they make up only a very small portion of
their natural diet. Much like the mechanical "bug zappers,"
bats and birds capture all manner of other flying insects
also. Efforts to colonize and conserve these animals should
not be done with the primary intent of diminishing biting
mosquitoes. When it comes to managing mosquitoes, a
good rule of thumb is if the approach or device sounds too
good to be true - it probably is.


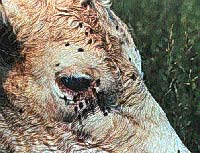 Frequent rains that have fallen over much of the state have
provided moist breeding areas needed by the flies that can
plague beef and dairy operations. House flies and stable
flies breed in wet mixtures of manure and spilled feed
around confinement areas or forage racks while face flies
and horn flies use fresh deposits of cow manure in
pastures. The longer breeding sites are able to stay moist,
the more flies they can produce. By mid-summer lower
humidity, air movement, and sunlight usually help to dry
these sites and make them less suitable for breeding. Our
steady parade of rains can play havoc with even the best
manure management and fly control plans.
Frequent rains that have fallen over much of the state have
provided moist breeding areas needed by the flies that can
plague beef and dairy operations. House flies and stable
flies breed in wet mixtures of manure and spilled feed
around confinement areas or forage racks while face flies
and horn flies use fresh deposits of cow manure in
pastures. The longer breeding sites are able to stay moist,
the more flies they can produce. By mid-summer lower
humidity, air movement, and sunlight usually help to dry
these sites and make them less suitable for breeding. Our
steady parade of rains can play havoc with even the best
manure management and fly control plans.
Sanitation and manure management around confinement areas is the key to breaking fly life cycles. In the long run, no reasonable amount of insecticide can overcome the breeding potential of optimum sites. Here are some things for confinement and pasture flies that can help-
Insecticides can be important tools to temporarily reduce or knock back fly problems until sanitation problems can be addressed but they are not be a satisfactory long term solution. While adults are the target of most of these programs, hitting the breeding sites is the real key.
Check the 2004 Insect Management Recommendations for the appropriate livestock species to see what products are registered for use.
For more information livestock pests, visit "Insect Management Recommendations".


Recent diagnostic samples have included Pythium root rot on alfalfa and on soybean; head scab on wheat; angular leaf spot, blue mold, target spot, black shank, Pythium root rot, tomato spotted wilt virus, manganese toxicity and temporary phosphorus deficiency on tobacco.
On fruit and vegetable samples, we have seen anthracnose and black rot on grape; anthracnose on blueberry; brown rot on cherry; early blight, bacterial speck, Septoria leaf spot, Rhizoctonia stem rot, and tomato spotted wilt virus on tomato; bacterial spot on pepper; and stinkbug injury on sweet corn.
On ornamentals and turf, we have seen anthracnose on
begonia; stalk borer on lily; black root rot on petunia;
Phytophthora aerial blight, black root rot and Rhizoctonia
root rot on vinca; yellow poplar weevil injury on tuliptree
and magnolia; black spot on rose; leaf blister on oak;
anthracnose, brown patch and Drechslera leaf spot on
ryegrass; and melting out on bluegrass.


UKREC-Princeton, KY, June 18-25, 2004| Black Cutworm
| 5
| True Armyworm
| 57
| Corn Earworm
| 3
| European Corn Borer
| 2
| Southwestern Corn Borer
| 0
| | |
To view previous trap counts for Fulton County, Kentucky go to - http://ces.ca.uky.edu/fulton/anr/ and click on "Insect Trap Counts".
For information on trap counts in southern Illinois visit the Hines Report at - http://www.ipm.uiuc.edu/pubs/hines_report/index.html. The Hines Report is posted weekly by Ron Hines, Senior Research Specialist, at the University of Illinois Dixon Springs Agricultural Center
NOTE: Trade names are used to simplify the information presented in this newsletter. No endorsement by the Cooperative Extension Service is intended, nor is criticism implied of similar products that are not named.
Lee Townsend
Extension Entomologist