Kentucky Pest News Newsletter
HIGHLIGHTS IN THIS ISSUE
Number 1029__________July 12, 2004
CORN
SOYBEAN
VEGETABLES
FRUIT
SHADE TREES AND ORNAMENTALS
PESTS OF HUMANS
MISCELLANEOUS
DIAGNOSTIC LAB HIGHLIGHTS
IPM TRAP COUNTS

CORN
DRY SOILS COULD SPELL TROUBLE IN CORN FIELDS WITH ROOT ROTS
By Paul Vincelli
 Many corn fields experienced long periods of wet soils
after planting. When soils are generally wet to saturated
early in the season, the corn plant produces a shallower
root system than in seasons when periods of drying take
place. Compounding this situation is the fact that root rots
have been diagnosed in a number of instances in Kentucky
corn fields, principally root rot caused by the fungus
Fusarium verticillioides (formally known as Fusarium
moniliforme, the same fungus that can produce fumonisins
in the grain). Root rot caused by F. verticillioides is
typically triggered by some stress to the plant, including
herbicide injuries. Pythium root rot is favored by soil
saturation, so fields with poor drainage (either poor
surface drainage or poor internal soil drainage) are prone
to this problem.
Many corn fields experienced long periods of wet soils
after planting. When soils are generally wet to saturated
early in the season, the corn plant produces a shallower
root system than in seasons when periods of drying take
place. Compounding this situation is the fact that root rots
have been diagnosed in a number of instances in Kentucky
corn fields, principally root rot caused by the fungus
Fusarium verticillioides (formally known as Fusarium
moniliforme, the same fungus that can produce fumonisins
in the grain). Root rot caused by F. verticillioides is
typically triggered by some stress to the plant, including
herbicide injuries. Pythium root rot is favored by soil
saturation, so fields with poor drainage (either poor
surface drainage or poor internal soil drainage) are prone
to this problem.
Fields of corn with root systems that are shallow due to
post-planting rains and damaged by root-rotting infections
will have a difficult time filling grain should weather
conditions turn dry. All one can do at this stage is adopt a
wait-and-see attitude. However, for fields that dry down
quickly during harvest, it would be valuable to review the
overall production system for stresses: the
appropriateness of hybrid selection, the weed control
program, degree of soil compaction, fertility, etc. The UK
Extension publication, A Comprehensive Guide to Corn
Management, ID-139, is an excellent resource for such a
purpose.
For information about corn pests, visit
"Insect Management Recommendations".


SOYBEAN
COULD SOYBEAN RUST BE IMPORTED INTO THE UNITED STATES WITH SEED, BULK GRAIN OR MEAL?
By Don Hershman
 Few scientists I have talked with, or heard speak, believe
agricultural commerce will be the means by which
soybean rust (SBR) makes its way into the continental
United States. Having said this, everyone I have heard
speak on the topic also feels it is not an impossible
scenario.
Few scientists I have talked with, or heard speak, believe
agricultural commerce will be the means by which
soybean rust (SBR) makes its way into the continental
United States. Having said this, everyone I have heard
speak on the topic also feels it is not an impossible
scenario.
The SBR pathogen, Phakopsora pachyrhizi, is not seed-borne,
but it could infest trash in seed and bulk grain. This,
however, is a remote possibility for a variety of reasons.
First, soybean is harvested AFTER plants drop their leaves.
Thus, the vast majority of rust-infected leaves will be on
the ground when the crop is harvested. Secondly, even if
some dead leaves with rust make there way into harvested
seed/grain, the chances are very high that the fragile
urediniospores of P. pachyrhizi would die soon after
harvest. Finally, it is my understanding that seed and bulk
grain produced in Brazil are almost always processed
through cleaners and dryers following harvest. This
further reduces the chances that viable rust spores will
contaminate those products. Of course, meal is even
further processed, compared to seed and bulk grain.
On the other hand, SBR can be present in all above-ground
plant parts, including pods and stems. Thus, if a seed lot or
bulk grain shipment is contaminated with SBR in pods or
stem pieces, the risk for bringing in P. pachyrhizi with seed
and grain may be increased. I say may, because I really do
not know. This type of information - specifically, the
survival characteristics of urediniospores of P. pachyrhizi -
have been studied very little.
At present, there are no published data indicating how
long Phakopsora pachyrhizi urediniospores could survive in
a shipment of soybean seed, grain or meal. At a conference
in St. Louis hosted by the American Soybean Association
this past January, I heard that it takes a minimum of 60
days for a bulk grain shipment of soybean to make its way
into a U.S. port following harvest. The period for seed
could be significantly shorter. Under normal
circumstances, the SBR fungus would not be expected to
survive in trash for this length of time. A concern was
raised at the meeting that urediniospores might remain
viable in containment longer than we think because of the
higher moisture level and lack of light in the hull of a
cargo ship. There is a great need for research to be
conducted in this area.
In the meantime, officials with the Animal and Plant
Health Inspection Service (APHIS) are on high alert and
are closely scrutinizing each shipment of soybean whose
port of origin is a county with known infestation of SBR.
When I searched the World Wide Web on this topic, it
became clear that there is a great deal of unrest and
concern by all parties involved that we do everything
possible to keep SBR from being imported with moving
seed, grain, and meal shipments.
Most scientists at this time are still convinced that SBR will
naturally make its way into this country as a result of
wind-blown urediniospores from South America, or
perhaps in the winds of a hurricane system originating
over Africa.
I will conclude with a statement made in a February 23,
2004 USDA-APHIS report entitled "Status of Scientific
Evidence on Risks Associated with the Introduction into
the Continental United States of Phakopsora pachyrhizi With
Imported Soybean Grain, Seed, and Meal":
"Soybean leaf debris associated with "foreign material" found in
soybean grain presents a theoretical pathway for the
introduction of SBR. However, normal commercial practices
minimize the presence of "foreign material" to less than 2%.
Moreover, as it is a normal commercial practice to harvest
soybeans after the plants have been defoliated, leaf debris should
compose only a fraction of the "foreign material"; therefore,
making "foreign material" found in grain an unlikely pathway
for introduction of SBR."
For more information about soybean pests, visit
"Insect Management Recommendations".


FRUIT
GREEN JUNE BEETLE AND FRUIT CROPS
By Ric Bessin
 As fruit crops begin to ripen across the state, one insect
pest is almost certain to cause problems, the green June
beetle. Unlike the Japanese beetle that is primarily a leaf
feeder, although it will attack damaged fruit and sound
fruit on occasion, the green June beetle almost exclusively
feeds on the fruit. It may be easier to list fruit that this pest
does not attack as most of the fruit crops are vulnerable.
Growers of peaches, apples, grapes, blueberries, and
blackberries have regular battles with this pest.
As fruit crops begin to ripen across the state, one insect
pest is almost certain to cause problems, the green June
beetle. Unlike the Japanese beetle that is primarily a leaf
feeder, although it will attack damaged fruit and sound
fruit on occasion, the green June beetle almost exclusively
feeds on the fruit. It may be easier to list fruit that this pest
does not attack as most of the fruit crops are vulnerable.
Growers of peaches, apples, grapes, blueberries, and
blackberries have regular battles with this pest.
Green June beetle is attracted to ripening fruit often in the
last few days before maturity. This is when the sugar
content of the fruit begins to peak. Damage by green June
beetle often attracts other insect harvest pests including
sap beetle, Japanese beetles, fruit flies, wasps and bees.
Control of green June beetle is not easy. Although we have
very effective sprays that can eliminate the pest, the
difficulty is timing. They typically arrive in the last few
days before harvest begins. The required pre-harvest
interval (PHI) of the more effective sprays is longer than
period before harvest will begin, so they cannot be used
when the beetles arrive. Growers often will substitute a
less effective material with a short pre-harvest interval in
order to comply with the required waiting period.
Sanitation is also very important, not only for green June
beetle, but the other pests that are attracted to the
damaged fruit and the scent of the fermenting plant juices.
To the extent the practical and possible, the damaged fruit
needs to be removed from the orchard/vineyard. In
intense situations or where organic-certification precludes
the use of synthetic insecticides, netting can be used over
small plants and vines as a barrier to the beetles.

VEGETABLES
FUNGICIDE FAILURES IN COMMERCIAL VEGETABLE CROPS
By William Nesmith
When I started my professional career with commercial
vegetable producers 34 years ago, a long-standing,
successful Virginia vegetable grower told me this. "Teach
them that water is critical to vegetable production. In dry
seasons they need timely irrigation, but when Mother
Nature supplies it in wet seasons, they will need timely
fungicide sprays ahead of the rains." In my opinion, that
advice is as fitting today as it was in 1970.
The protracted periods of rainy weather this spring have
led to a number of serious disease outbreaks in
commercial vegetable crops. Even growers with much past
success are having serious problems in disease
management. On most new vegetable farms, the inoculum
load has been low previously, allowing some to escape
serious disease in recent years and get by with less than
good spray programs for a few years. Such, have not
learned earlier of the need to incorporate a strong disease
management program in their operation. At a recent field
day, I reminded the audience that one must always give
luck its due credit in assessing management issues - until a
disease is critical, the critical value of controlling it is not
visible! A poor spray program in situations of low disease
pressures can be adequate. Such luck can guide one to
decide that recommended spray programs are not as
important as others have advised, leading to inadequate
equipment, omitted or reduced sprays, and lack of needed
products in the supply system. Some experienced sages
have even been hurt by relying too heavily on newer
products and extending spray intervals rather a using
them in a rotational pattern with proven preventive
products and closer spray intervals. Long spray intervals
look real good to the bottom-line in dry weather, but some
are seeing a very different bottom line (in red) from those
same programs in this wet season.
Vine crops are especially hard hit on some farms from
outbreaks of anthracnose, gummy stem blight, Alternaria
leaf spots, and scab even before we enter the season for
Phytophthora blight, powdery mildews and downy
mildews. By the way, expect an earlier than normal season
for all three diseases and at much higher levels than
normal.
Although a number of other issues are involved, such as
site selection, rotation, and infested transplants, with the
operations I have reviewed, fungicide application
difficulties seem central to the problems. Some growers
just have not used much fungicide. Some were waiting to
see if the disease would become serious enough to warrant
spraying. Others have not used them often enough,
mainly relying on the schedules used in drier seasons then
trying to "chase" the outbreak rather than preventing it.
Some have been waiting for it to stop raining so they could
get into the field; those need to realize that heavy grass-
sod spray-strips have a place in our soils. Some have
abandoned wet areas of the field and left it unsprayed for
the pathogens to use as a staging area; those could have
helped themselves with either prompt destruction or some
spot spraying with back-pack equipment. Abandoning a
portion of the field is leaving it for the pest to colonize.
Others that apply a tank mixture of fungicides and
insecticides have been afraid to spray during the day time
for fear of killing their bees (a major concern) so they have
been waiting for dark, yet by late in the day and at dark
thunderstorms have moved in, often night after night.
Separate the fungicide and insecticide application in such
weather, because you need that fungicide on and dried
before the rain, with morning applications often being the
best options to insure drying for the wet event. Some have
selected the wrong fungicides for the problems present.
Fungicides are important tools for controlling infectious
diseases in commercial vegetable crops. Chemical
fungicides work mainly as preventives but some newer
eradicants (curatives) have moved into the system.
However, where curatives are available they need to be
used in a preventive manner, rather than always relying
on that curative potential, by either applying in a tank
mixture or a rotation pattern with other effective products.
Plan to control by inhibiting subsequent infections of the
pathogen rather than by curing the diseased plant, because
the pathogens have very short reproductive cycles and
build their numbers very rapidly, in a matter of days.
Application timing is very important relative to when the
infection and sporulation events are occurring in disease
development. For most fungicides, applications should be
made before the disease even begins, or no later than when
the first few lesions appear. Why? Because these materials
stop the pathogens by preventing spore germination and
preventing subsequent infections and not by
killing/eradication of the pathogen after it is already
inside the plant. Most infections occur while the leaf is wet,
so the fungicide needs to be on target tissue and dried
before the moisture event. Failure to appreciate this
concept has been central to most of the decision making in
problem fields that I have encountered. Yes, there is a lot
to do in a day, and it is difficult to find time to do it, but
disease prevention measures must take a higher priority in
some seasons than others. Wet seasons are disease
seasons!
Good spray coverage is a critical issue with fungicide use.
Protective fungicides should be applied to achieve good
coverage for the best protection under strong disease
pressure. Although some products are formulated to be
less sensitive to coverage issues than others, the goal for all
should be good coverage put in place and dried before the
wet events.
Spray adjuvants or surfactants should be used if the
product label recommends them to ensure uniform
coverage. Do not use these materials unless the label
indicates they are needed.
It is important to maintain proper application intervals
with fungicides, especially in wet weather. If it rains before
the material is dry on the foliage, most of the fungicide
will wash off. Yet infection occurs while the plants are
wet-when the protective chemical is most needed! If it
seems likely that the fungicide can remain on the foliage
for two hours or more before a rain, it is best to go ahead
and make an application. This is particularly true if the
spray interval has been long and/or a protracted weather
event is involved. In addition, regular applications are
needed to keep up with new plant growth and loss of the
applied material due to weathering. With the fast growth
rates experienced this season, it is not uncommon to have
one or two feet of new growth that has no fungicide
between applications. If inoculum is in the field, that
foliage has probably already become infected.


SHADE TREES AND ORNAMENTALS
PINE WILT NEMATODE ACTIVE IN KENTUCKY
By John Hartman
Scots and Austrian pines in Kentucky landscapes and
along highways are now showing symptoms of pine wilt
disease caused by the pine wood nematode
(Bursaphelenchus xylophilus). Although we see the disease
mainly on Scots pines, Austrian pines are also susceptible,
and on rare occasions white pines show the disease. In
some cases, the disease completes the demise of trees
already infected with pine tip blight disease.
Symptoms. The first visible symptom is discoloration of
needles from green to yellow to brown. These symptoms
are accompanied by a marked decrease in resin flow that is
apparent when a branch is cut and resin does not seep
readily from the wound. Wilt and death of affected trees
may occur gradually or very rapidly. Brown trees can be
seen now within groups of pines and they contrast
markedly with nearby heathy green trees. Trees infected
in the spring may wilt and die by late summer.
Spread. Long-horned cerambycid beetles carry the
nematodes on and in their bodies as they emerge from
infected (usually dead) trees. Beetles then migrate to
nearby pines where nematodes enter healthy shoots
through feeding wounds left by the beetles. They migrate
into resin canals where they rapidly multiply and cause
the pines to wilt and die. Dying pines are attractive to the
beetles for breeding; the cycle then repeats.
Sampling for diagnosis. Positive diagnosis of this disease
requires microscopic identification of the nematode. A
recently wilted or killed pine can be sampled by collecting
the portion of an affected branch closest to the trunk. A
good sample might consist of a branch section 1/2 inch or
more in diameter, and about a foot long. Alternatively,
samples consisting of sapwood can be taken from the
trunk or main branches using a hatchet or an increment
borer. It is best to collect several samples from various
parts of the tree. Put the wood samples in a plastic bag
and submit them to the Plant Disease Diagnostic Lab via
the County Extension Office.
Disease management. If pine wilt nematode disease is
confirmed in a planting of pines, remove and destroy the
affected trees. This measure will help prevent spread of
the disease to nearby healthy pines. Control of the insect
vector with insecticide treatments has not proven to be a
useful tool for managing pine wilt disease. Under unusual
experimental conditions, the pinewood nematode was
shown to move from fresh infected pine wood chips to
young Scots pines. Thus, it is probably a good idea to
compost fresh chips from pine wilt-infested trees for at
least one month before using them as mulch around
susceptible pine species.


Category
CHIGGERS- JUST SCRATCHING THE SURFACE
By Lee Townsend
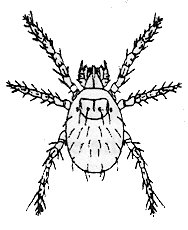 Chigger bites, a sure sign of summer, can be the "souvenir"
of a blackberry-picking trip, working, or playing in
overgrown brushy or grassy areas. Chiggers usually feed
where clothing fits tightly against the skin - waistbands,
etc. Digestive juices used by the mites to dissolve skin cells
cause bite sites to become red and swollen. Later, angry
red welts will form that itch intensely for several days. As
if that were not enough, bite sites can become infected if
they are scratched incessantly.
Chigger bites, a sure sign of summer, can be the "souvenir"
of a blackberry-picking trip, working, or playing in
overgrown brushy or grassy areas. Chiggers usually feed
where clothing fits tightly against the skin - waistbands,
etc. Digestive juices used by the mites to dissolve skin cells
cause bite sites to become red and swollen. Later, angry
red welts will form that itch intensely for several days. As
if that were not enough, bite sites can become infected if
they are scratched incessantly.
Here are some ways to protect yourself-
Wear long pants tucked into boots or socks to keep
chiggers on the outside of your clothing Wear loose fitting
clothing and avoiding sitting or lying directly on the
ground.
Avoid walking through overgrown fields and brush,
especially from July through early September. Instead,
walk in the center of mowed trails to avoid vegetation
where chiggers (and ticks) congregate.
Use an insect/ tick repellent. Products containing diethyl
toluamide (DEET) or permethrin (clothing only) are most
effective. Be sure to read and follow directions for use on
the container. A hot, soapy shower immediately after
coming indoors will remove chiggers that have not yet
attached.
Chigger management
Control of chigger infestations in large yards, parks,
camps, picnic sites, and other recreational areas is often
impractical. However, chiggers in play and picnic areas
and around trails can be reduced by vegetation
management. Regular mowing and brush removal creates
a less favorable habitat for chiggers and the rodents and
other small animals on which they feed. This is the way to
a long-term solution.
Insecticide sprays may provide some temporary reduction
of chiggers. They are most effective when directed into
areas where chiggers and their animal hosts are likely to
frequent. Options include bifenthrin (Ortho Lawn Insect
Killer), carbaryl, (Sevin), cyfluthrin (Bayer Advanced Lawn
and Garden Multi-Insect Killer, and any of a number of
products containing permethrin. Be sure to read the
product label carefully to be sure the site you are planning
to treat is on the label. Also, look for specific instructions
for applications against chiggers that can increase control.


DIAGNOSTIC LAB HIGHLIGHTS
DIAGNOSTIC LAB - HIGHLIGHTS
By Julie Beale and Paul Bachi
Recent samples in the Diagnostic lab have included
stinkbug injury, zinc deficiency, and Fusarium stalk rot on
corn; black shank, blue mold, frogeye leaf spot; soreshin,
Fusarium basal stem canker, black root rot, Rhizoctonia
leaf blight, Fusarium wilt; tomato spotted wilt virus,
manganese toxicity, hollow stalk and weather fleck
(ozone) on tobacco.
On fruits and vegetables, we have diagnosed Fusicoccum
canker on blueberry; anthracnose, bitter rot, black rot and
potash deficiency on grape; bitter rot, cedar-apple rust,
fireblight, frogeye, Pythium root rot on apple; brown rot
on peach; Entomosporium leaf spot on pear; Blumeriella
leaf spot on cherry; anthracnose on walnut; anthracnose
and Rhizoctonia stem rot on bean; and Fusarium wilt,
early blight, bacterial speck and spot; Septoria leaf spot
and Cladosporium leaf mold on tomato.
On ornamentals and turf, we have seen southern blight on
hosta; Pythium root rot and manganese deficiency on
chrysanthemum; Rhizoctonia stem rot and Pythium root
rot on petunia; powdery mildew on dogwood; scab on
crabapple; Actinopelte leaf spot on oak; Phomopsis twig
blight on juniper; Verticillium wilt on smoketree;
Cercospora leaf spot on willow; and summer patch on turf.


IPM TRAP COUNTS:
By Patty Lucas, University of Kentucky Research Center
UKREC-Princeton, KY, July 2-9, 2004
| Black Cutworm
| 0
|
| True Armyworm
| 18
|
| Corn Earworm
| 21
|
| European Corn Borer
| 1
|
| Southwestern Corn Borer
| 27
|
|
To view previous trap counts for Fulton County, Kentucky
go to - http://ces.ca.uky.edu/fulton/anr/
and click on "Insect Trap Counts".
For information on trap counts in southern Illinois visit the
Hines Report at -
http://www.ipm.uiuc.edu/pubs/hines_report/index.html.
The Hines Report is posted weekly by Ron Hines, Senior
Research Specialist, at the University of Illinois Dixon
Springs Agricultural Center
MISCELLANEOUS
LARGE "BUGS OF SUMMER"
By Lee Townsend
Several of Kentucky's largest or most unusual insects will
be active over the next few weeks. A few species that are
most likely to appear and at least arouse curiosity are:
 The eastern Hercules beetle (Dynastes tityus) has a large
(2" to 2-1/2" long) greenish-gray to black body. Males have
a large distinctive horn on the head and are sometimes
called rhinoceros beetles. The adults are attracted to lights
during mid- to late summer. The larvae are large white
grubs that feed in the center of decaying logs and stumps.
It is the largest beetle in Kentucky and is harmless. These
beetles occur throughout the southeastern United States.
The eastern Hercules beetle (Dynastes tityus) has a large
(2" to 2-1/2" long) greenish-gray to black body. Males have
a large distinctive horn on the head and are sometimes
called rhinoceros beetles. The adults are attracted to lights
during mid- to late summer. The larvae are large white
grubs that feed in the center of decaying logs and stumps.
It is the largest beetle in Kentucky and is harmless. These
beetles occur throughout the southeastern United States.
 Dobsonflies are large, prehistoric-looking insects. They
have soft gray bodies and two pair of wings with a
network of veins. These fluttery-flying insects are usually
found near creeks or streams. The male has an impressive
pair of long, slender mouthparts. Females have the same
body shape but very small mouthparts.
Dobsonflies are large, prehistoric-looking insects. They
have soft gray bodies and two pair of wings with a
network of veins. These fluttery-flying insects are usually
found near creeks or streams. The male has an impressive
pair of long, slender mouthparts. Females have the same
body shape but very small mouthparts.
The female dobsonflies lay their eggs on overhanging
branches or structures over streams. The larvae called
"hellgrammites" usually occur under stone in streams
where they feed on insects that live in the water. The
larvae live for several years in the water and are used as
bait by fishermen. The adults only live a few days to
reproduce, then die.
- Giant water bugs have a variety of common manes as
"toe biters", "fish killers", and "electric-light bugs". They are
large (about 2.5 inches long) with flat, brown, oval bodies,
large eyes and a pair of grabbing front legs. These insects
are good fliers and can show up as uninvited guests in
swimming pools. These water bugs live in quiet standing
water of ponds, lakes, and stream pools, where they feed
on aquatic insects, snails, tadpoles, and even minnows.
While not dangerous, they can inflict painful bite if
handled.
- The European hornet, about 1.5 inches long, is an
impressive and fearsome-looking insect. It is brown with
yellow markings, particularly at the end of the abdomen.
European hornets generally nest in cavities in trees or
other protected sites. They will fly at night and are
attracted to lights. More information is available in Entfact
600 - The European Hornet in Kentucky.
http://www.uky.edu/Agriculture/Entomology/entfacts/struct/ef600.htm
 The cicada killer wasp (about 2 inches long) can be seen
cruising over grassy areas during July and August. They
are black with yellow markings and rusty-colored wings.
These wasps burrow into sandy or well-drained soil with
sparse grass color. They live in individual burrows but
where conditions are right, large groups can build up over
time. They are curious but not aggressive. The females can
sting and will if handled but do not respond like
yellowjackets or other common social wasps.
The cicada killer wasp (about 2 inches long) can be seen
cruising over grassy areas during July and August. They
are black with yellow markings and rusty-colored wings.
These wasps burrow into sandy or well-drained soil with
sparse grass color. They live in individual burrows but
where conditions are right, large groups can build up over
time. They are curious but not aggressive. The females can
sting and will if handled but do not respond like
yellowjackets or other common social wasps.

NOTE: Trade names are used to simplify the information presented in
this newsletter. No endorsement by the Cooperative Extension Service
is intended, nor is criticism implied of similar products that are not
named.
Lee Townsend
Extension Entomologist
BACK
TO KY PEST NEWS HOME
 Many corn fields experienced long periods of wet soils
after planting. When soils are generally wet to saturated
early in the season, the corn plant produces a shallower
root system than in seasons when periods of drying take
place. Compounding this situation is the fact that root rots
have been diagnosed in a number of instances in Kentucky
corn fields, principally root rot caused by the fungus
Fusarium verticillioides (formally known as Fusarium
moniliforme, the same fungus that can produce fumonisins
in the grain). Root rot caused by F. verticillioides is
typically triggered by some stress to the plant, including
herbicide injuries. Pythium root rot is favored by soil
saturation, so fields with poor drainage (either poor
surface drainage or poor internal soil drainage) are prone
to this problem.
Many corn fields experienced long periods of wet soils
after planting. When soils are generally wet to saturated
early in the season, the corn plant produces a shallower
root system than in seasons when periods of drying take
place. Compounding this situation is the fact that root rots
have been diagnosed in a number of instances in Kentucky
corn fields, principally root rot caused by the fungus
Fusarium verticillioides (formally known as Fusarium
moniliforme, the same fungus that can produce fumonisins
in the grain). Root rot caused by F. verticillioides is
typically triggered by some stress to the plant, including
herbicide injuries. Pythium root rot is favored by soil
saturation, so fields with poor drainage (either poor
surface drainage or poor internal soil drainage) are prone
to this problem.




 As fruit crops begin to ripen across the state, one insect
pest is almost certain to cause problems, the green June
beetle. Unlike the Japanese beetle that is primarily a leaf
feeder, although it will attack damaged fruit and sound
fruit on occasion, the green June beetle almost exclusively
feeds on the fruit. It may be easier to list fruit that this pest
does not attack as most of the fruit crops are vulnerable.
Growers of peaches, apples, grapes, blueberries, and
blackberries have regular battles with this pest.
As fruit crops begin to ripen across the state, one insect
pest is almost certain to cause problems, the green June
beetle. Unlike the Japanese beetle that is primarily a leaf
feeder, although it will attack damaged fruit and sound
fruit on occasion, the green June beetle almost exclusively
feeds on the fruit. It may be easier to list fruit that this pest
does not attack as most of the fruit crops are vulnerable.
Growers of peaches, apples, grapes, blueberries, and
blackberries have regular battles with this pest.


 Chigger bites, a sure sign of summer, can be the "souvenir"
of a blackberry-picking trip, working, or playing in
overgrown brushy or grassy areas. Chiggers usually feed
where clothing fits tightly against the skin - waistbands,
etc. Digestive juices used by the mites to dissolve skin cells
cause bite sites to become red and swollen. Later, angry
red welts will form that itch intensely for several days. As
if that were not enough, bite sites can become infected if
they are scratched incessantly.
Chigger bites, a sure sign of summer, can be the "souvenir"
of a blackberry-picking trip, working, or playing in
overgrown brushy or grassy areas. Chiggers usually feed
where clothing fits tightly against the skin - waistbands,
etc. Digestive juices used by the mites to dissolve skin cells
cause bite sites to become red and swollen. Later, angry
red welts will form that itch intensely for several days. As
if that were not enough, bite sites can become infected if
they are scratched incessantly.


 The eastern Hercules beetle (Dynastes tityus) has a large
(2" to 2-1/2" long) greenish-gray to black body. Males have
a large distinctive horn on the head and are sometimes
called rhinoceros beetles. The adults are attracted to lights
during mid- to late summer. The larvae are large white
grubs that feed in the center of decaying logs and stumps.
It is the largest beetle in Kentucky and is harmless. These
beetles occur throughout the southeastern United States.
The eastern Hercules beetle (Dynastes tityus) has a large
(2" to 2-1/2" long) greenish-gray to black body. Males have
a large distinctive horn on the head and are sometimes
called rhinoceros beetles. The adults are attracted to lights
during mid- to late summer. The larvae are large white
grubs that feed in the center of decaying logs and stumps.
It is the largest beetle in Kentucky and is harmless. These
beetles occur throughout the southeastern United States.
 Dobsonflies are large, prehistoric-looking insects. They
have soft gray bodies and two pair of wings with a
network of veins. These fluttery-flying insects are usually
found near creeks or streams. The male has an impressive
pair of long, slender mouthparts. Females have the same
body shape but very small mouthparts.
Dobsonflies are large, prehistoric-looking insects. They
have soft gray bodies and two pair of wings with a
network of veins. These fluttery-flying insects are usually
found near creeks or streams. The male has an impressive
pair of long, slender mouthparts. Females have the same
body shape but very small mouthparts.
 The cicada killer wasp (about 2 inches long) can be seen
cruising over grassy areas during July and August. They
are black with yellow markings and rusty-colored wings.
These wasps burrow into sandy or well-drained soil with
sparse grass color. They live in individual burrows but
where conditions are right, large groups can build up over
time. They are curious but not aggressive. The females can
sting and will if handled but do not respond like
yellowjackets or other common social wasps.
The cicada killer wasp (about 2 inches long) can be seen
cruising over grassy areas during July and August. They
are black with yellow markings and rusty-colored wings.
These wasps burrow into sandy or well-drained soil with
sparse grass color. They live in individual burrows but
where conditions are right, large groups can build up over
time. They are curious but not aggressive. The females can
sting and will if handled but do not respond like
yellowjackets or other common social wasps.