L-Canavanine:
A Potential Chemotherapeutic Agent For Human Pancreatic Cancer
Gerald
A. Rosenthal
Laboratory of Biochemical Ecology
, University of Kentucky, Lexington, KY 40506
ABSTRACT
L-Canavanine, the principal nonprotein
amino acid of certain leguminous plants, is a potent L-arginine antimetabolite.
This natural product has demonstrative antineoplastic activity against
a number of human cancers. Recent studies with MIAPaCa-2 and CFPAC have
established canavanine's potential anticancer potential against these human
pancreatic adenocarcinomas. Canavanine has promise as a lead compound in
the development of a chemotherapeutic agent for the treatment of human
pancreatic carcinoma, but it has not been adequately investigated. Greater
study of canavanine and its derivatives is needed to fully realize the
experimental and therapeutic value of this naturally-occurring non-protein
amino acid, and to obtain a chemotherapeutic agent of clinical value in
treating human carcinomas.
INTRODUCTION
L-Canavanine is a potent arginine antimetabolite
that bears strong structural analogy to its protein amino acid counterpart:
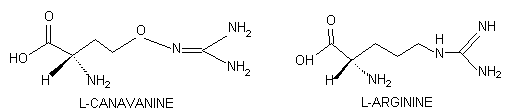
Replacement of the terminal methylene
group of arginine with oxygen produces this nonprotein amino acid distinguished
by a guanidinooxy moiety that has a pKa value of 7.04 and an
isoelectric point near neutrality (Boyar and Marsh, 1982). The pKa
of 10.48 for the guanidino group of L-arginine produces a much more basic
amino acid with Io = 12.48 (Greenstein and Winitz, 1963). Therefore,
at physiological conditions, arginine is essentially fully protonated whereas
canavanine is not.
As a subtle structural mimic of L-arginine,
canavanine can function in all enzymic reactions for which arginine is
a substrate (Rosenthal, 1977). Therefore, canavanine potentially can inhibit
any enzyme-directed reaction employing arginine as the preferred substrate.
Arguably, canavanine's most adverse effect results from its activation
and aminoacylation to the cognate tRNAArg by the arginyl-tRNA
synthetase of canavanine-sensitive organisms (Allende and Allende, 1964;
Mitra and Mehler, 1967). Incorporated into the nascent polypeptide chain,
the decreased basicity of canavanine relative to arginine can affect residue
interaction and thereby disrupt the tertiary and/or quaternary interactions
essential for establishing the requisite three dimensional conformation
of a given protein.
Biochemical Basis
for Canavanine's Antimetabolic Properties
To determine the biochemical and biological
consequence of canavanyl protein formation, a number of canavanine-containing
proteins, obtained from various insect sources, were examined in detail.
In the initial study, canavanine was provided to gravid females of the
locust, Locusta migratoria migratorioides [Orthoptera], whose ovarian
mass had been removed surgically on the day of adult emergence. This surgical
procedure resulted in a pronounced accumulation of vitellogenin, an important
hemolymph storage protein. Analysis of canavanyl vitellogenin, purified
from hemolymph of these canavanine-treated locusts, shows that on average
18 of the 200 arginyl residues of vitellogenin or about 1 in 225 amino
acids are replaced by canavanine. This modest level of replacement is nevertheless
sufficient to elicit a dramatic alteration in protein structure that is
shown most effectively by electrophoretic analysis. The altered fragmentation
pattern of canavanyl vitellogenin, relative to the native macromolecule,
undoubtedly reflects the profound change in vitellogenin structure resulting
from canavanine incorporation (Rosenthal et al., 1989a).
These disparate forms of vitellogenin
were also treated with chemicals able to react with surface-exposed amino
acid residues to form novel amino acids. For example, treatment with cyanate
carbamylates reactive lysyl residues and converts them to homocitrullyl.
About one quarter of the lysyl residues of native vitellogenin do not react
with cyanate; presumably, these are inaccessible and buried within the
interior of the macromolecule. These residues are readily carbamylated
in canavanyl vitellogenin. A similar chemical approach establishes that
nearly twice as many surface-exposed tyrosine residues are acetylated as
compared to the native protein. These experiments establish that canavanine
incorporation into vitellogenin alters the three dimensional conformation
of the protein, a property essential for normal function (Rosenthal et
al., 1989a).
Microbial infection or mechanical injury
to larvae of the fly, Phormia terranovae [Diptera] induces a family
of protective, antibacterial proteins known trivially as the diptericins
(Keppi et al., 1986). If canavanine is provided at the time of mechanically
injury, it is incorporated into all of these protective proteins (Rosenthal
et al., 1989b). This assimilation causes a total loss of detectable
antibacterial activity for nearly all the diptericins; only a single diptericin—diptericin
A retains demonstrable biological activity. This insectan investigation
provides compelling experimental evidence that canavanine incorporation
into a protein can impair protein function.
Canavanine-mediated impairment in function
was also demonstrated with a catalytic protein, lysozyme (EC 3.2.1.17),
that is induced by injection of fragments of the cell wall of Micrococcus
lutea into larvae of the tobacco hornworm, Manduca sexta [Sphingidae].
If these larvae are provided canavanine when challenged, it is readily
incorporated into de novo-synthesized lysozyme. The ratio of canavanine
to arginine in this aberrant lysozyme is 1:3.8; assay of canavanyl lysozyme
discloses a 48% loss in catalytic activity. This study provided the first
demonstration of the ability of canavanine, through aberrant protein formation,
to adversely affect the catalytic activity of an enzyme.
The importance of aberrant protein formation
in the expression of canavanine's antimetabolic effect is also supported
convincingly by a radically different experimental approach that determines
how frequently canavanine replaces arginine in the proteins of various
insects, i.e. the substitution error frequency (SEF). Manduca sexta
larvae incorporate about 3.5% of the administered radiolabeled canavanine
into hemolymphic proteins after 24 h. This results in a SEF that varies
according to the insectan tissue but is about I in 2 for proteins of the
larval body wall and musculature (Rosenthal et al., 1987). The bruchid
beetle, Caryedes brasiliensis [Coleoptera], an inhabitant
of the neotropic forests of Costa Rica, develops from larva to adult in
the canavanine-laden seeds of Dioclea megacarpa [Fabaceae]. The
woody pericarp of D. megacarpa houses seeds that can contain as
much as 13% canavanine by dry weight (Rosenthal, 1983). The weevil, Sternechus
tuberculatus [Curculionidae], oviposits on the pericarp of Canavalia
brasiliensis [Fabaceae]; larvae, foraging within the fruit, are sustained
by seeds that also store appreciable canavanine. These two seed predators
are examples of insects that are adapted biochemically to canavanine (Bleiler
et al., 1988) and can therefore tolerate, even flourish with this
normally poisonous natural product (Rosenthal, 1983). The SEF for C.
brasiliensis is 1 in 365 while that of S. tuberculatus
is 1 in 500-1,000. The tobacco budworm, Heliothis virescens [Noctuidae],
does not consume canavanine-containing plants; however, it is naturally
resistant to this potentially toxic allelochemical (Berge et al., 1986;
Melangeli et al., 1997). This aggressive generalist herbivore exhibits
a SEF of 1 in 65. Thus, canavanine-adapted and canavanine-resistant insects
minimize or avoid canavanyl protein formation while canavanine-sensitive
insects readily incorporated this arginine antagonist.
Article of Human
Diet
At present, few canavanine-containing
seeds are part of the human diet, but this is changing as the worldwide
demand for reasonably priced, high quality protein increases. Jack bean
seeds, Canavalia ensiformis (L.) DC. [Fabaceael, which contain
about 2.5% canavanine by dry weight, and sword bean, Canavalia gladiata
[Fabaceae], storing about 1.4% canavanine by dry weight (Rosenthal
and Nkomo, 1997), are important table legumes, particularly in Asia and
parts of the tropics and Africa. In North America, the most heavily consumed
canavanine-containing plant is alfalfa, Medicago sativa [Fabaceae].
Canavanine is the preponderant nonprotein amino acid of the seed where
it accounts for 1.5% of the dry matter; the sprout is also canavanine-rich
since it can accumulate as much as 2.4% canavanine by dry weight (Rosenthal
and Nkomo, 1997).
Canavanine's Antineoplastic
Activity
In 1958, Kruse and McCoy reported that
canavanine competed with arginine in meeting the growth requirements of
Walker carcinosarcoma 256 cells. In a subsequent study, Kruse et al.
(1959) demonstrated that canavanine was incorporated into the proteins
of these cancer cells, and that the diminution in the amount of arginine
in the protein hydrolysate equaled the canavanine content. This
report established for the first time that canavanine specifically replaced
arginine in de novo-synthesized tumor proteins. Schachtele and Rogers
(1965) employed the same experimental approach to demonstrate the incorporation
of canavanine into the proteins of Escherichia coli. These initial
observations were confirmed by experiments conducted with M. sexta
larvae that were injected with L-[guanidinooxy-14C]canavanine
(Dahlman and Rosenthal, 1976). Enzymatic degradation of the radiolabeled
canavanine, isolated from the insectan hydrolysate, demonstrated unequivocally
that the hydrolysate 14carbon was derived from radiolabeled
canavanine.
Numerous descriptive studies have documented
that exposure of a particular organism to canavanine adversely affected
a basic property or functional parameter of one or more of its enzymes.
For example, the normally soluble
b-galactosidase
of E. coli exhibited diminished activity, and sedimented readily
with the 10,000 xg pellet after exposing the bacterium to canavanine (Prouty
et al., 1972; Rosenthal, 1977).
Several studies of canavanine's antineoplastic
activity have been conducted. Naha et al. (1980) demonstrated that
canavanine selectively inhibited DNA replication in epithelial monkey kidney
cells possessing a transformed phenotype, as compared to their counterpart
that remained "contact-inhibited" with a temperature change from 33 to
39.5oC.
A detailed study of canavanine's antineoplastic
activity was conducted with mice bearing L1210 leukemic cells (Green
et al., 1980). These workers reported that DNA synthesis was reduced
to only 9% of the control level, as assessed by [3H]thymidine
incorporation, after 12-hourly i.p. injections of 20 mg canavanine per
injection. A dramatic attenuation in DNA synthesis of 86% of the control
level was also achieved when the infected mice were infused s.c. with canavanine
continuously for 1 d at a rate of 20 mg h-1 after an initial
i.p. injection of 20 mg canavanine. At an optimal dose of 18 g kg-1,
the median life span of the cancerous mice increased by 44%. This investigation
was of great significance because it demonstrated that canavanine could
mediate its toxic effect not only at the level of protein function, but
also through its ability to disrupt DNA replication.
In an important follow-up study, Green
and Ward (1983) reported that canavanine enhanced significantly the efficacy
of g -irradiation of cultured HT-29 cells, a human tumor cell line. The
lethal effect of this radiation was augmented both when canavanine was
provided prior to as well as after g -irradiation. These workers provided
convincing experimental evidence for their contention that canavanine's
lethal effect was manifested preferentially in rapidly proliferating cells—a
property often essential for chemotherapeutic efficacy. The experimental
efforts of Green and his collaborators did not distinguish between the
possibility that canavanine affected nucleic acid turnover directly as
compared to its acting by affecting the activity of one or more proteins
essential to maintaining DNA replication.
Canavanine also affected the growth
of a rat colonic carcinoma in male Fischer rats (Thomas et al.,
1986). This tumor, which became palpable in 8-10 d, had a doubling time
of 3 to 4 d. Canavanine, administered by s.c. injection into the flank
opposite the tumor site, was provided initially after the tumor attained
a volume of 500-1,000 m3 (Figure 1). Providing 2.0 g kg-1
canavanine for 5 d produce a tumor vs. control of 23%; after 9 d, this
value fell to 14%. Canavanine's efficacy was enhanced when the dose was
increased to 3.0 g kg-1. At this dose, the percentage of regression
was –13% after 5 d, and –8% after 9 d. These negative values reflect tumor
regression. The loss in tumor volume, expressed as percentage of regression,
was 22% for the 3 g kg-1 daily for 5 d, and 60% in the 3 g kg-1
daily for 9 d treatment groups.
These promising findings with canavanine
had the drawback that the treated rats lost weight. Canavanine's cumulative
toxicity resulted in about a 15% diminution in body weight after 5 treatment
days. This finding led to an examination of the relationship of caloric
deprivation to tumor growth reduction, and established that canavanine-directed
curtailment of tumor growth was not caused by reduced food intake. Most
importantly, canavanine-dependent weight loss was fully reversible. This
investigation instigated efforts to develop canavanine derivatives with
an enhanced therapeutic index while diminishing body weight loss.
Canavanine's Cytotoxic
Effect On Human Pancreatic Cells
Toxicological study of canavanine metabolism
in the male Fischer rat revealed that L-[guanidinooxy-14C]canavanine
was assimilated preferentially by proteins of the pancreas. Radiolabeled
canavanine incorporation into these proteins was 10-times that of liver,
brain, and muscle tissues, and 5-times that of the proteins of most other
body organs (Thomas and Rosenthal, 1987a). Given the pronounced assimilation
of canavanine into pancreatic proteins, we examined the effect of canavanine
on the growth of MIAPaCa-2, a human pancreatic adenocarcinorna cell line
(Swaffar et al., 1994). When these cells were grown in Dulbecco's
modified Eagle's medium (DMEM) containing 0.4 mM arginine, the 50% inhibitory
concentration (IC50) for canavanine was 2 mM. Most importantly,
the IC50 value fell precipitously to 10.0 µM when the
arginine content of the culture medium was reduced to 0.4 µM.
This study was important because it
demonstrated canavanine's marked cytotoxic activity against a human pancreatic
cancer cell line. Moreover, it provided an excellent experimental system
for evaluating novel canavanine derivatives on a small scale prior to conducting
whole animal studies. Swaffar et al. (1994) also demonstrated that
canavanine-mediated inhibition of MIAPaCa-2 cell growth was reversible
by arginine up to 12 hr post treatment, but then became irreversible. This
finding, while not presently explicable, was important because it demonstrated
that conditions existed where canavanine's anti-cancer effect would not
be reversed.
Combination Therapy
Having established canavanine's antineoplastic
activity against MIAPaCa-2 cells, Swaffer et al. (1995) asked the
salient question of how canavanine functioned in combination with 5-fluorouracil
(5-FU), the preferred drug presently utilized for treating pancreatic carcinoma.
In this companion study, canavanine's efficacy as a chemotherapeutic agent
was assessed in combination with 5-FU. At a fixed molar ratio of 1:1, over
the range of 0.06 to 1.0 mM, both drugs exhibited greater cytotoxic effects
against MIAPaCa-2 cells relative to single administration of these drugs.
The IC50 values for canavanine and 5-FU were 0.060 and 0.363,
respectively; in combination, the IC50 value fell to 0.021.
As part of this investigation, the interaction
of canavanine and 5-FU was also assessed with the colonic carcinoma of
adult male Fischer rats described above (Swaffar et al., 1995).
Providing canavanine, either at 1.0 g kg-1 or 2.0 g kg-1
daily for 5 d in combination with 5-FU increased significantly the anti-tumor
activity of either drug alone (Figure 2).
These investigations demonstrated the
value of employing canavanine in combination with drugs presently employed
in treating pancreatic and colonic cancer. Thus, the development of a canavanine
derivative as an effective drug is not limited only to its independent
use, but also may prove more valuable when provided in combination with
an established anticancer drug.
Canavanine's Accumulative
Toxicity
Analysis of canavanine catabolism in
the adult rat demonstrated that hepatic argjnase (EC 3.4.1.5) fostered
the hydrolysis of L-canavanine to yield L-canaline and urea, this reaction
pathway was the principal basis for canavanine catabolism in this mammal
(Thomas and Rosenthal, 1987b).
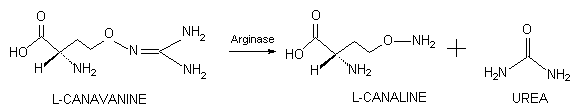
Thus, it is reasonable to propose that
administration of L-canavanine to a human would result in the formation
of L-canaline, a highly toxic nonprotein amino acid that is a powerful
inhibitor of pyridoxal phosphate-dependent enzymes. Rahiala et al
(1971) were the first to recognize that a direct reaction occurred between
canaline and the vitamin B6 moiety of an enzyme by postulating:
"...that canaline probably inhibits pyridoxal phosphate-containing enzymes
by its nonenzymic, irreversible and stoichiometric binding with pyridoxal
phosphate."
The ability of canaline to inactivate
an enzyme by forming a stable, covalently-linked oxime was demonstrated'
directly with L-[U-14C]canaline which reacted with ornithine
aminotransferase (EC 2.6.1.13) of larval Manduca sexta to yield
an enzyme-bound, radiolabeled canaline-pyridoxal phosphate oxime (Rosenthal
and Dahlman, 1990). In addition, canaline's facile ability to form oximes
means that canaline can scavenge such essential 2-oxo-containing metabolites
as pyruvate, oxaloacetate, and 2-oxoglutarate to deplete carboxylic acid
reserves and carbon skeleton required for amino acid synthesis. Finally,
canaline also possesses significant antineoplastic and antiproliferative
properties (Rosenthal, 1998).
Canavanine Derivatives
As Chemotherapeutic Agents
The intrinsic toxicity of canavanine
would be decreased significantly if it failed to function as a substrate
for hepatic degradation via the action of arginine. Production of a simple
ester of canavanine, such as methyl-L-canavanine, provided a derivative
that was not an effective substrate for rat arginase and therefore could
not elicit the adverse biological effects caused by canaline. In addition,
this ester possessed enhanced hydrophobicity relative to canavanine, and
might therefore enjoy enhanced cellular uptake. Intracellular esterases
should release the parent compound through hydrolysis and thereby increase
canavanine's bioavailability.
To test this hypothesis, the methy,
ethyl, n-propyl, isopropyl, n-butyl, and n-octyl esters
of L-canavanine were synthesized and evaluated for cytotoxicity against
MIAPaCa-2 cells (NaPhuket et al., 1997). While the methyl, ethyl
n-propyl, and isopropyl esters of canavanine exhibited slightly
improved growth-inhibitory activity against MIAPaCa-2 cells, the n-butyl
and n-octyl esters of canavanine dramatically attenuated cellular
growth (Figure 3). This conclusion was firmly confirmed by bioevaluation
of the growth inhibiting properties of these canavanine derivatives in
terminal instar M. sexta larvae (Rosenthal et al., 1997).
The increased potency of these latter esters may reflect their enhanced
lipophilicity and greater membrane penetrational properties. None of the
free alcohols, except n-octanol, possessed significant growth-inhibiting
activity. Only at concentrations above 2.5 mM did octanol exhibit significant
activity against MIAPaCa-2 cells.
Conclusions
The experimental efforts outlined in
this review detail studies that demonstrate the significant antineoplastic
activity of canavanine. Recently completed experiments probing 14C-radiolabeled
canavanine uptake (0.26% of the administered dose) by MIAPaCa-2 cells revealed
that 70% of the cellular canavanine was found in the proteins, the remainder
was accounted for in the cytosol; 95% of the 14carbon found
in proteins of the treated cells was canavanine (NaPhuket, 1997). In contrast,
only 58% of the 14carbon of the cytosol was canavanine.
The author raises for thought the interesting
possibility that canavanine's antineoplastic activity might arise from
the formation of structurally aberrant, dysfunctional, canavanine-containing
proteins by the cancer cells and that they may be unique to these cells.
The findings outlined in this review support the contention that canavanine
has marked potential as a lead compound in the development of a chemotherapeutic
agent for the treatment of human pancreatic carcinoma. Particularly noteworthy
are certain ester derivatives of canavanine, which might provide an efficacious
drug capable of eliciting little if any body weight loss while enhancing
the therapeutic index. Esterification of the carboxyl group of canavanine
with longer-chained alcohols such as butanol and octanol represent structural
modification of canavanine that augments significantly the growth-inhibiting
properties of the parent compound against MIAPaCa-2 cells.
Further study of canavanine and its
derivatives could lead to chemotherapeutic agents of clinical value in
treating human carcinomas. Additional experimental effort is warranted
to realize the experimental and therapeutic value of this unusual amino
acid antimetabolite of higher plants.
REFERENCES
Allende, C.C., and Allende, J. E. (1964).
Purification and substrate specificity of arginyl-ribonucleic acid synthetase
from rat liver. J. Biol. Chem. 239:1102-1106.
Berge, M.A., Rosenthal, G.A., and Dahlman,
D.L (1986). Tobacco budworm, Heliothis virescens [Noctuidae] resistance
to L-canavanine, a protective allelochemical. Pest. Biochem. Physiol.
25:319-326.
Bleiler, J., Rosenthal, G.A., and Janzen,
D. H. (1988). Biochemical ecology of canavanine-eating seed predators.
Ecol. 69:427-433.
Boyar, A., and Marsh, R. E. (1982).
L-Canavanine, a paradigm for the structures of substituted guanidines.
J. Am. Chem. Soc. 104:1995-1998.
Dahlman, D.L., and Rosenthal, G.A.
(1976). Further studies on the effect of L-canavanine on the tobacco
hornworm, Manduca sexta (L.) (Sphingidae) J. Insect Physiol.
22:265-271.
Green, M.H., Brooks, T.L., Mendelsohn,
J., and Howell, S.B., (1980). Antitumor activity of L-canavanine against
L1210 murine leukemia. Cancer Res. 40: 535-537.
Green, M. H., and Ward, J. F. (1983).
Cancer Res. Enhancement of human tumor cell killing by L-canavanine
in combination with g -radiation. Cancer Res. 43: 4180-4182.
Greenstein, J.P., and Winitz, M. (1963).
Chemistry of the Amino Acids, Vols. 1-3, Wiley & Sons, New York.
Keppi, E., Zachary, D., Robertson, M.,
Hoffmann, D., and Hoffmann, J. (1986). Induced antibacterial proteins in
the hemolymph of Phormia terranovae [Diptera]. Purification and
possible origin of one protein. Insect Biochem. 16:395-399.
Kruse, Jr., P.F., and McCoy, T.A.
(1958). The competitive effect of canavanine on utilization of arginine
in growth of Walker carcinosarcoma 256 cells in vitro. Cancer Res. 18:279-282.
Kruse, Jr., P.F., White, P.B., Carter,
H.A.,.and McCoy, T.A. (1959). Incorporation of canavanine into protein
of Walker carcinosarcoma 256 cells cultured in vitro. Cancer Res. 19:122-125.
Melangeli, C., Rosenthal, G.A, and
Dahlman, D.L. (1997). The biochemical basis for the tolerance of Heliothis
virescens to L-canavanine Proc. NatI. Acad. Sci. USA 94:2255-2260.
Mitra, S.K., and Mahler, A.H. (1967).
The arginyl transfer ribonucleic acid synthetase of Escherichia coli.
J. Biol. Chem. 242:5490-5494.
Naha, P.M., Silcock, J. M., and Fellows,
L (1980). An experimental model for selective inhibition of proliferating
cells by chemotherapeutic agents. Cell Biol. Inter Rept. 4: 155-166.
NaPhuket, S. L-Canavanine and its
Derivatives as Potential Chemotherapeutic Agents for Pancreatic Cancer:
Synthesis, Structure-Activity Relationships And Metabolic Studies. Ph.D.
Thesis, University of Kentucky, 1997.
NaPhuket, S.,Trifonov, L.S., Crooks.
P.A., Rosenthal, G.A., Freeman, J.W., and Strodel W.E. (1997). Synthesis
and structure-activity relationships of some antitumor congeners of L-canavanine.
Drug Devel. Res. 40:325-332.
Prouty, W.F., Karnovsky, M.J., and Goldberg,
A.L. (1972). Degradation of abnormal proteins in Escherichia coli.
J. Biol. Chem. 250:1112-1122.
Rahiala, E.-L., Kekomaki, M.K., Janne,
J., Raina, A., and Raiha, N.C.R., (1971). Inhibition of pyridoxal enzymes
by L-canaline Biochim. biophys. Acta 227:337-343.
Rosenthal, G.A. (1977). The biological
effects and mode of action of L-canavanine, a structural analogue of L-arginine.
Q. Rev. Biol. 52:155-178.
Rosenthal, G.A. (1983). The adaptation
of a beetle to a poisonous plant. Sci. Amer. 249:164-171.
Rosenthal, G.A., and Dahlman, D.L.,
(1990). Interaction of L-canaline with ornithine aminotransferase of the
tobacco hornworm, Manduca sexta [Sphingidae]. J. Biol.
Chem. 265:868-873
Rosenthal, G.A., and Nkomo, P. (1997).
Unpublished data.
Rosenthal, G.A., Lambert, J., and Hoffmann,
D. (1989a). L-Canavanine incorporation into protein can impair macromolecular
function. J. Biol. Chem. 264:9768-9771.
Rosenthal, G.A., Reichhart, J.-M., and
Hoffmann, J.A. (1989b). L-Canavanine incorporation into vitellogenin and
macromolecular conformation. J. Biol. Chem. 264: 13693-13696.
Rosenthal, G.A., Berge, M.A., Bleiler,
J.A., and Rudd, T. (1987). Avoidance of aberrant protein production and
an organism's ability to utilize or tolerate L-canavanine. Experientia
43:558-561.
Schachtele, C.F., and Rogers, P.
(1965). Canavanine death in Escherichia coli. J. Mol. Biol. 34:843-860.
Swaffar, D.S., Ang, C.Y., Desai,
P.B., and Rosenthal, G.A. (1994). Inhibition of the growth of human
pancreatic cancer cells by the arginine antimetabolite, L-canavanine. Cancer
Research 54:6045-6048.
Swaffar, D.S., Choo, Y.A.., Desai,
P.B., Rosenthal, G.A., Thomas, D.A., John, W.J., and Crooks, P.A., (1995).
Combination therapy with 5-flurouracil and L-canavanine: in vitro
and in vivo studies. Anti-Cancer Res. 6:586-593
Thomas, D.A., Rosenthal, G.A., Gold,
D.V., and Dickey, K. (1986). Growth inhibition of a rat colon tumor by
L-canavanine. Cancer Research 46:2898-2903
Thomas, D.A., and Rosenthal, G.A.,
(1987a) Toxicity and pharmacokinetics of the nonprotein amino acid
L-canavanine in the rat. Toxicology & Appl. Pharm. 91:395-405.
Thomas, D.A., and Rosenthal, G.A., (1987b)
Metabolism of L-[guanidinooxy-14C]-canavanine in the rat. Toxicology
& App/. Pharm. 91:406-414.
LEGEND TO THE FIGURES
Figure 1. The effect of canavanine
on tumor growth in male Fischer 344 rats. The rats were administered canavanine,
2.0 ( ), 3-0 ( ) g kg-1 for 9 d.
Control animals ( ) received 0.95% (w/v) NaCl. The standard
error bar was omitted if it fell within the area occupied by the data point,
n=5 + SEM.
Figure 2. Evaluation of the combined
effect of canavanine and 5-FU on colonic tumor growth in male Fischer rats.
Tumor growth was evaluated after 5 daily s.c. injections of: 1.0 g kg-1
canavanine ( ) 2.0 g kg-1, canavanine (
) 35 mg kg-1 5-FU ( ) 35 mg kg-1 5-FU
+ 1.0 g kg-1 canavanine ( ) and 35 mg kg-1
5-FU + 2.0 g kg-1 canavanine ( ). The control animals
( ) received 0.95% (w/v) NaCl. Each value is the mean (n = 5) +
SEM.
Figure 3. Comparison of the IC50
value for canavanine and some of its esters.

