PROFESSOR
GERALD A. ROSENTHAL
has been temporarily reopened to provide access
to his final publication
Gerald A. Rosenthal
Laboratory of Biochemical Ecology, Scottsdale, Arizona 85262-7112
Summary
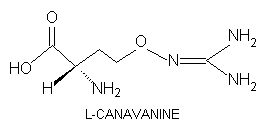
L-Canavanine,
L-2-amino-4-(guanidinooxy)butyric
acid, is a potentially toxic nonprotein amino acid of certain leguminous
plants. Many species are prolific canavanine producers; they divert enormous
nitrogen resource to the storage of this single natural product. Canavanine,
a highly effective protective allelochemical, provides a formidable chemical
barrier to predation and disease.
The accumulated experimental
evidence leaves little doubt that the key element in the ability of canavanine
to function as an effective protective allelochemical is its subtle structural
mimicry of arginine which makes it an effective substrate for amino acid
activation and aminoacylation, and its marked diminution in basicity relative
to arginine which mediates the production of structural aberrant, dysfunctional
canavanyl proteins.
The biological burdens of
canavanyl protein formation by canavanine-treated Manduca sexta larvae
were carried throughout their remaining life cycle. Protein-based sequestration
of canavanine prevented turnover and clearance of the free amino acid,
and undoubtedly contributed significantly to the antimetabolic character
of this protective allelochemical.
INTRODUCTION
L-Canavanine, L-2-amino-4(guanidinooxy)butyric acid, is a non-protein amino acid synthesized by leguminous plants that are members of the Lotoidea, a subfamily of the Leguminosae (1). Many canavanine-synthesizing legumes store prodigious amounts of this nonprotein amino acid: members of the genus Canavalia typically commit 3-4% of their seed dry matter to canavanine storage (2); the neotropical legume, Dioclea megacarpa, sequesters so much seed canavanine that this single metabolite commanders more than 95% of every nitrogen atom allocated to free amino acid production (3). Colutea arborescens, Caragana arborescens, Vicia gigantea, Robinia pseudoacacia and Wisteria floribunda, representative of many prolific canavanine producers, store from nearly 6 to 13% canavanine by dry weight (3). The purpose of this review is to probe the rationale for plant investment of so much nitrogen into a single secondary metabolite.
CANAVANINE CHEMISTRY
L-Canavanine bears marked structural analogy to L-arginine in that the terminal methylene group of arginine is replaced with oxygen.

Oxygen is significantly more electronegative than
carbon; this causes enhanced electron withdrawal that facilitates deprotonation,
and reduces the pKa value of the guanidinooxy group to about
7.04 (4)—far less than 12.48,
the pKa of the guanidino group of
arginine (5). Under physiological conditions, arginine, a highly basic
amino acid, is fully protonated while canavanine is
much more anionic. This marked difference in
the charge state of the R group
of canavanine, as compared to that of arginine,
can disrupt critical R group interactions within a given protein and affect
profoundly the way in which the protein folds into its three-dimensional
conformation. Alteration in essential conformation can affect adversely
protein function.
CANAVANYL PROTEIN FORMATION AND FUNCTION
The structural analogy between
L-canavanine
and L-arginine is so marked that this arginine mimic
serves as a substrate in virtually every enzyme-mediated reaction that
preferentially employs arginine as a substrate. This structural subtlety
is nowhere more important than with arginyl tRNA synthetase, which readily
esterifies L-canavanine to the cognate tRNAArg
(6,7).
One of the defining hallmarks of all canavanine-sensitive insects is an
inability to differentiate between these amino acids; these organisms readily
activate canavanine and incorporates it into their de novo-synthesized
proteins in place of arginine (8).
The bruchid beetle, Caryedes
brasiliensis, oviposits on the canavanine-laden seeds of Dioclea
megacarpa which typically contain 8-9% canavanine by dry weight (9).
The developing weevil, Sternechus tuberculatus, feeds on seeds of
the legume, Canavalia brasiliensis, where canavanine levels can
reach 6-8% of the dry matter (8). These neotropical seed predators possess
highly discriminatory arginyl tRNA synthetases that permits them to scrupulously
avoid canavanine activation and esterification (10).
The tobacco hornworm, Manduca
sexta [Sphingidae] feeds on solanaceous plants, none of which has the
genomic complement for canavanine production. Therefore, there has not
been an opportunity for evolutionary adaptation to this toxic allelochemical.
Numerous experimental studies have fully documented the potent insecticidal
properties of canavanine against this lepidopteran plant feeder (11-13).
This arginine antagonist markedly reduces overall larval growth; delays
and otherwise disrupts larval-pupal and pupal-adult
ecdysis; produces profound larval, pupal, and adult growth defects; elicits
severe diuresis; and dramatically attentuates female fucundity and fertility.
Typically, canavanine-treated larvae that survive the larval instars, expire
in a futile attempt at pupal-adult metamorphosis or the body parts of the
adult are so severely malformed as to be nonfunctional (14). Manduca
sexta larval sensitivity to canavanine markedly increases when the
canavanine dietary concentration exceeds 2.5 mM or they receive more than
1.0 mg/g larval body weight by parenteral injection.
Under optimal conditions for
canavanine incorporation, 3.0% of the administered L-[guanidinooxy-14C]canavanine
is incorporated into newly synthesized hemolymphic proteins after 24
h (15) [Figure 1]. Analysis of the radiolabeled body wall (the thoracic
musculature and integument ) proteins discloses the unexpected and important
finding that on average one of two arginyl residues are replaced with canavanine
(15).
Insert Figure one
Manduca sexta larvae possess a highly effective system for preferential degradation of canavanine-containing proteins relative to their normal counterpart (16). This finding was established by preparing L-[guanidinooxy-14C]canavanine- and L-[guanidino-3H]arginine-labeled hemolymphic proteins that were purified of free radiolabeled amino acids, filter-sterilized, and reintroduced into the hemolymph of receipient larvae (16) [Figure 2]. This metabolic capacity may result in a significant "underestimation" of the actual level of canavanine incorporated into insectan proteins after 24 hr.
Insert Figure two
Insight into the biochemical
basis for M. sexta
sensitivity to canavanine can be gained by examining
the effect of canavanine incorporation on de novo-synthesized insectan
proteins. Several independent lines of experimental evidence including:
electrophoretic analysis of native and canavanyl vitellogenin, probing
conformational differences by evaluating surface-exposed amino acid residues,
and monoclonal antibody analyses established that canavanine incorporation
into the vitellogenin of the gravid locust, Locusta migratoria migratorioides
[Acrididae]
profoundly alters the unique three-dimensional structure (17).
Other experiments, conducted
with third instar larvae of the meat-eating fly, Phormia terranovae
[Diptera],
demonstrated that canavanine assimilation into their protective, antibacterial
proteins, known trivially as diptericins A, B or C and peak V protein,
nullified the protective efficacy of all of the antibacterial proteins
except diptericin A whose biological activity was significantly impaired
(18) [Figure 3].
Insert Figure three
Finally, lysozyme, induced in M. sexta larvae in the presence of canavanine, had 21% of its arginyl residues replaced by canavanine. This substitution caused a loss of nearly one-half of the catalytic activity of this protein (19). These independent and detailed investigations provided the first experimental evidence linking canavanyl protein formation to the loss of essential protein function.
D-CANAVANINE AND MANDUCA SEXTA
D-Canavanine is biologically active in M. sexta larvae, e.g. it causes larval edema—a characteristic consequence of canavanine consumption. Significantly, the D-enantiomer, which is not a substrate for arginyl tRNA synthetase, shows little of the adverse larval growth effects and pupal deformation that are hallmarks of canavanine toxicosis and which have been linked consistently to aberrant, canavanyl protein formation (20). As postulated, divorced from a role in protein synthesis, the D-enantiomer does not elicit any of the symptomology linked to the formation of structurally anomalous, conformationally altered, and functionally impaired canavanine-containing proteins.
HOMOARGININE AND CANAVANINE TOXICITY
Uptake studies, employing L-[guanidino-14C]homoarginine,
established that this arginine antagonist is readily assimilated into the
primary protein pools of the developing M. sexta larva: hemolymph
proteins as well as those of the body wall and musculature. Administration
of homoarginine to M. sexta larvae, at 1.5 times the canavanine
dose that proved lethal to all tested larvae within 48 h, failed to elicit
any adverse larval growth or developmental effects; all larvae successfully
ecdysed to pupae and then adults (21).
A study designed to evaluate
the effect on lysozyme activity of replacing arginine with various arginine
analogues revealed that L-homoarginine did not disrupt
lysozyme activity [Figure 4] (21). In a similar vein, assimilation of homoarginine
into the diptericins of P. terranovae had no discernible effect
on their antibacterial activity [Figure 5], homoarginine is incorporated
as effectively as canavanine into larval proteins without adversely affecting
larval growth and development as well as enzymic function. Why?
It is my contention that homoarginine
is innocuous because of the elevated pKa value for its guanidino
group–arguably higher than that of arginine (21). Being at least as basic
as arginine, homoarginine is incorporated into insectan proteins without
disrupting R group interaction and essential protein conformation. In summary,
the innocuous nature of homoarginine as compared to canavanine probably
results from the far greater basicity of the former. The accumulated experimental
evidence continues to lend credence to the concept that the key element
in the ability of canavanine to function as an effective protective allelochemical
is its subtle structural mimicry of arginine which makes it an effective
substrate for amino acid activation and aminoacylation, and its decreased
basicity relative to arginine which mediates the production of structural
aberrant, dysfunctional canavanyl proteins.
In a recent study, we administred
L-[guanidinooxy-14C]canavanine
to terminal instar larvae by a single parenteral injection and then evaluated
the presence of radiolabeled canavanine in newly synthesized larval proteins.
We subsequently isolated the proteins of the pupae or adults that developed
from comparably radiolabeled larvae, and discovered no significant diminution
in the amount of [14C]canavanine/mg protein. Thus, the biological
burdens of canavanyl protein formation to canavanine-treated larvae were
carried throughout their remaining life cycle. This protein-based sequestration
of canavanine prevented its turnover and clearance, and undoubtedly contributed
significantly to the antimetabolic character of this protective allelochemical
(22)
CANAVANINE ESTERIFICATION
Reasoning that converting canvanine to its simple esters would enhanced its hydrophobicity and hopefully increase its penetration into the cell membrane, we evaluated the biological effects of the methyl- and ethyl-ester of canavanine on M. sexta larval growth (22). Since the ethyl ester proved more toxic than than the methyl ester, we synthesized even longer-chained esters, specifically the propyl, butyl, isobutyl, and octyl esters of L-canavanine and evaluated their insecticidal properties (22). Of the tested esters, the isobutyl and octyl esters were far more deleterious to larval growth and development; a single injection of the isobutyl or octyl ester was lethal to all of the test animals before the termination of the larval instar. This deleterious effect was not caused by the release of the free alcohol, via the action of an insectan esterase, but rather related directly to esterification of the parent compound (22).
HELIOTHIS VIRESCENS: AN INSECT REMARKABLY RESISTANT TO CANAVANINE
Terminal instar larvae of the
tobacco budworm,
Heliothis virescens [Noctuidae], a highly destructive
agricultural pest, reared on canavanine-containing diet had an LC50
value for this nonprotein amino acid of 300 mM (23). This LC50
corresponds to 53,000 ppm wet diet weight or nearly 40% on a dry weight
basis. While the pupae that emerged from such treated larvae were depauperate,
they lacked any discernible developmental aberrations. A group of five
larvae reared on a staggering 500 mM canavanine-containing diet survived
for 9 days before the onset of larval death.
Analysis of canavanine consumption
established that the treated larvae neither eliminate signficant canavanine
in their fecal matter nor sequester it within an internal body organ (24).
Rather, the larvae efficiently metabolize canavanine with a t1/2 of
135 min (24). Several lines of experimental evidence demonstrated that
the larvae do not induce a detoxification enzyme in response to canavanine
exposure, rather its ability to metabolize this normally potent toxicant
is constituted (24).
Administration of L-[guanidinooxy-14C]canavanine
to terminal instar larvae generated [14C]guanidine as the principal
in
vivo larval radiolabeled catabolite (25). This finding implicated a
larval reductase that fosters the reduction of canavanine to homoserine
and guanidine. Independent experiments, involving H. virescens larvae,
led to the discovery of another larval enzyme—a reductase that catalyzes
an NADH-dependent reduction of hydroxyguanidine to guanidine (26). This
discovery immediately suggested that canavanine may be catabolized initially
to homoserine and hydroxyguanidine rather than guanidine, via a novel hydrolase
able to cleave the O-N bond of the guanidinooxy moiety of the substrate.
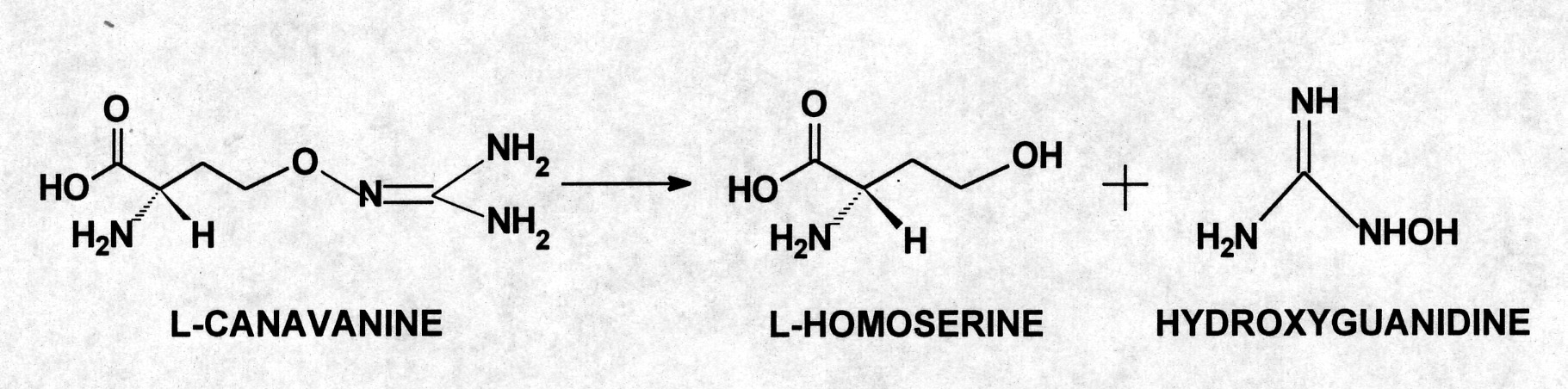
Although this catabolic reaction had been observed in a soiL-borne Pseudomonas (27), the responsible enzyme was never isolated nor had this metabolic capacity been described previously from an eukaryotic organism. Overall, the experimental evidence implicated two gut enzymes that were functioning in concert to metabolize canavanine. The first reaction used a novel hydrolase that directed the formation of L-homoserine and hydroxyguanidine from L-canavanine. In the second phase, hydroxyguanidine was reduced to guanidine.
CANAVANINE HYDROLASE
The search for this enzyme
in the gut of larval
H. virescens culminated in the isolation of
a homogeneous enzyme that mediated an irreversible hydrolysis of L-canavanine
to L-homoserine and hyroxyguanidine (28). The existence
of canavanine hydrolase, an enzyme able to cleave an oxygen-nitrogen bond,
was an important finding since this enzyme is the only protein known to
demonstrate this catalytic ability. As such, it represents a new type of
hydrolase-one able to act on oxygen-nitrogn bonds that has been given the
novel designation: EC 3.13.1.1.
Canavanine hydrolase (EC 3.13.1.1)
exhibits a high affinity for canavanine as evinced by its apparent Km
value of 1.1 mM; the turnover number for this reaction is 21.1 mmol min-1.
This enzyme exhibits a high degree of substrate specificity as it cannot
function effectively with L-2-amino-5(guanidinooxy)pentanoate
nor 2-amino-3(guanidinooxy)propionate, the higher or lower homolog, respective
of L-canavanine nor with its methyl ester. Nor can
canavanine hydrolase react with L-canaline in a comparable
reaction that would have produced homoserine and hydroxylamine (28).
Insert three
The canavanine detoxification
pathway in H. virescens is different from that exhibited by larvae
of the canavanine-adapted burchid beetle, C. brasiliensis. The larvae
of this neotropical insect hydrolytically cleave L-canavanine
to L-canaline and urea (9). Urea was degraded subsequently
to ammonia to provide ammoniacal nitrogen for the production of insectan
amino acids (29). Indeed, it is this same arginase-mediated deguanidination
of L-canavanine to generate L-canaline
and urea, coupled with urease-driven hydrolysis of the latter to create
ammonia, that permits higher plants to effectively mobilize the stored
nitrogen of canavanine (30).
A thorough search of the relevant
literature revealed that many plants that are members of the Lotoideae
are consumed by larval
H. virescens (31,32). It is reasonable to
speculate that tobacco budworm larvae, feeding amongst canavanine-containing
plants, enhanced their natural resistance to this potentially toxic allelochemical,
over evolutionary time, by directing the synthesis of a novel enzyme that
accounts for the remarkable resistance of this generalist herbivore to
canavanine. It is noteworthy that H. virescens larvae, which are
highly resistant to canavanine’s antimetabolic efffects, exhibit little
ability to incorporate radiolabeled canavanine into de novo-synthesized
larval proteins [Figure 1]. (16).
CONCLUSION
Higher plants produce a number of arginine analogs including the higher homolog, L-homoarginine; its lower homolog, 2-amino-4-guanidinobutyric acid; 5-hydroxy-L-homoarginine; L-indospicine (L-2-amino-6-amidinohexanoic acid), and 5-hydroxy-L-arginine (33).
Insert four
None of these natural products is as effective an arginine antimetabolite as canavanine. The selection pressure that favored the development of the genome for canavanine synthesis and storage was driven undoubtedly by the subtle structural similarity between canavanine and arginine and the appreciable reduction in the basicity of canavanine relative to arginine that was achieved by the replacement of a terminal methylene group with oxygen. These factors combined to create a highly effective antimetabolite that provides a high level of defensive efficacy against a wide array of insectan predators and pests.
ACKNOWLEDGMENTS-The research review in this publication was made possible by decades long support of the National Science Foundation and the National Institutes of Health. The guidance, support, and assistance of many colleagues and associates, and especially a career-long collaboration with Dr. Douglas Dahlman of the University of Kentucky are also gratefully acknowledged.
REFERENCES
4. Boyar, A., and Marsh, R.E. (1982). L-Canavanine, a paradigm for the structures of substituted guanidines. Journal of the American Chemical Society 104: 1995-1998.
5. Greenstein, J.P., and Winitz, M. (1963). Chemistry of the Amino Acids, Vols 1-3, John Wiley & Sons, Inc., New York.
6. Allende, C.C., and Allende, J.E. (1964). Purification and substrate specificity of arginyl-ribonucleic acid synthetase from rat liver. Journal of Biological Chemistry 239: 1102-1106.
7. Mitra, S.K., and Mehler, A.H. (1967). The arginyl transfer ribonucleic acid synthetase of Escherichia coli. Journal of Biological Chemistry 242: 5490-5494.
8. Bleiler, J., Rosenthal, G.A., and Janzen, D.H. (l988). Biochemical ecology of canavanine-eating seed predators. Ecology 69: 427-433.
9. Rosenthal, G.A. (1983). Biochemical adaptation of the bruchid beetle, Caryedes brasiliensis to a poisonous plant. Scientific American 249:164-171.
10. Rosenthal, G.A. (1991). Nonprotein amino acids as protective allelochemicals. In Herbivores: Their Interaction with Secondary Plant Metabolites, Rosenthal, G.A., and Berenbaum, M., eds., 2nd. Ed., Academic Press, San Diego, CA.
11. Dahlman, D.L., and Rosenthal, G.A. (1975). Non-protein amino acid-insect interactions. I. Growth effects and symptomology of L-canavanine consumption by the tobacco hornworm, Manduca sexta (L.). Comparative Biochemistry and Physiology 51A: 33-36.
12. Rosenthal, G.A., and Dahlman, D.L. (1975). Non-protein amino acid-insect interaction. II. Studies of the effects of the canaline-urea cycle amino acids on the grwoth and development of the tobacco hornworm, Manduca sexta (L.) (Sphingidae). Comparative Biochemistry and Physiology 52A: 105-108.
13. Dahlman, D.L., and Rosenthal, G.A. (1976). Further studies on the effect of L-canavanine on the tobacco hornworm, Manduca sexta (L.) (Sphingidae) Journal of Insect Physiology 22: 265-271.
14. Rosenthal, G.A. (1977). The biological effects and mode of action of L-canavanine, a structural analogue of L-arginine. Quarterly Review of Biology 52: 155-178.
15. Rosenthal, G.A., Berge, M.A., Bleiler, J.A., and Rudd, T.P. (1987). Aberrant, canavanyl protein formation and the ability to tolerate or utilize L-canavanine. Experientia 43: 558-561.
16. Rosenthal, G.A., and Dahlman, D.L. (1986). L-Canavanine and protein syntheis in the tobacco hornworm, Manduca sexta. Proceedings of the National Academy of Sciences. 83: 14-18.
17. Rosenthal, G.A., Reichhart, J.-M., and Hoffmann, J.A. (1989). L-Canavanine incorporation into vitellogenin and macromolecular conformation. Journal of Biological Chemistry 264: 13693-13696.
18. Rosenthal, G.A., Lambert, J., and Hoffmann, D. (1989). L-Canavanine incorporation into protein can impair macromolecular function. Journal of Biological Chemistry 264: 9768-9771.
19.Rosenthal, G.A., and Dahlman, D. L. (1991). Studies of L-canavanine incorporation into insectan lysozyme Journal of Biological Chemistry 266: 15684-15687.
20. Rosenthal, G.A., Dahlman, D.L., Crooks, P.A., Na Phuket, S., and Trifonov, L.S. (1995). Insecticidal properties of some derivatives of L-canavanine. Journal of Agricultural and Food Chemistry 43: 2728-2734.
21. Rosenthal, G.A., and Harper, L. (1996) L-Homoarginine studies provide insight into the antimetabolic properties of L-canavanine. Insect Biochemistry and Molecular Biology 26: 389-394.
22. Rosenthal, G.A., Palesa, N., and Dahlman, D.L. (1998). Effect of long-chained esters on the insecticidal properties of L-canavanine. Journal Agricultural and Food Chemistry. 46: 296-299.
23. Berge, M.A., Rosenthal, G.A., and Dahlman, D.L. (1986). Tobacco budworm, Heliothis virescens [Noctuidae] resistance to L-canavanine, a protective allelochemical. Pesticide Biochemistry and Physiology 25: 319-326.
24. Berge, M., and Rosenthal, G.A. (1990). Detoxification of L-canavanine by the tobacco budworm, Heliothis virescens [Noctuidae] Journal of Agricultural and Food Chemistry. 38: 2061-2065.
25. Berge, M., and Rosenthal, G.A. (1991). Metabolism of L-canavanine and L-canaline in the tobacco budworm, Heliothis virescens [Noctuidae]. Chemical Research in Toxicology. 4: 237-240.
26. Rosenthal, G.A. (1992). Radiochemical synthesis and colorimetric analysis of hydroxyguanidine. Biorganic Chemistry 20: 55-61.
27. Kalyankar, G.D., Ikawa, M., and Snell, A.A. (1958). The enzymatic cleavage of canavanine to homoserine and hydroxyguanidine. Journal of Biological Chemistry 233: 1175-1178
28. Michelangeli, C., Rosenthal, G.A., and Dahlman, D.L. (1997). The biochemical basis for L-canavanine tolerance by the tobacco budworm, Heliothis virescens (Noctuidae) Proceedings of the National Academy of Sciences. 94: 1293-1297.
29. Rosenthal, G.A., Hughes, C.G., and Janzen, D.H. (1982). L-Canavanine, a dietary nitrogen source for the seed predator, Caryedes brasiliensis. Science 217: 353-355.
30. Rosenthal, G.A. (1991). Metabolism of L-canavanine and L-canaline in leguminous plants. Plant Physiology 94: 67-70.
31. Pearson, O. (1958). The Insect Pests of Cotton in Tropical Africa, Commonweatlh Institute of Entomology, London, UK.
32. Zulucki, M.P., Daglish, G., Firempong, S. and Twine, P. (1986). Australian Journal of Zoology 34: 779-814.
33. Rosenthal, G.A. (1982). Plant Nonprotein Amino and Imino Acids. Biological, Biochemical, and Toxicological Properties, 279pp. Academic Press, San Diego, CA.
LEGEND TO THE FIGURES
Figure 1. Incorporation of L-[guanidinooxy-14C]canavanine into newly synthesized hemolymphic proteins of Manduca sexta ( ) or Heliothis virescens ( ) terminal instar larvae. See (16) for additional experimental details.
Figure 2. The relative stability of [14C]canavanine- and [3H]arginine-labeled hemolymphic proteins, expressed as a 14C to 3H ratio. The radiolabeled proteins were injected into receipient larvae, and evaluated at the indicated times (hr). See (16) for additional experimental details.
Figure 3. The antibacterial potency of native and canavanyl diptericin A. Biological activity was evaluated as a function of colony forming ability of Escherichia coli D31 in the presence of native ( ), or canavanyl ( ) diptericin A. Control cultures ( ) received protein serum albumin. See (18) for additional experimental details.
Figure 4. The effect of homoarginine or canavanine incorporation on lysozyme activity. See (21) for additional experimental details.
Figure 5. The biological activity of variously
substituted diptericin A. Biological activity was evaluated as a function
of colony forming ability of Escherichia coli D31 in the presence
of native ( ), canavanyl ( ), or homoarginyl ( ) diptericin A. Control
cultures ( ) received protein serum albumin. See (21) for additional experimental
details.