Naming errors (p. 15)
The second example of an antiaromatic compound is 1,4-dihydropyrazine, not 1,4-dihydropyridazine. Thanks to Neil Moorcroft for spotting this error.
The fourth example of an antiaromatic compound is cyclooctatetraene, not cycloctatetraene. Thanks to Sheena Mae Amit for spotting this error.
The left-hand compound on the bottom of the page is called tropone, not tropolone, and its systematic name is cycloheptatrienone, not cycloheptadienone. Thanks to Mario Martinez and Brian Smith for spotting these errors.
On p. 29, I say that nucleophilicity is measured in H2O, but on p. 57, I say that it is measured in MeOH. It turns out that both statements are correct. The commonly used Swain–Scott nucleophilicities were originally measured in H2O, but later, the scale was modified to use MeOH as solvent. Thanks to Dmitry Cheshkov for spotting this inconsistency.
Problems 1.4 to 1.7 on pp. 24 and 38 should be renumbered 1.5 to 1.8. Thanks to Hui Wang for spotting this error.
In the second line of the small-font paragraph on p. 22, the symbol for low energy should be G°, not ΔG°. Thanks to Tadashi Okuyama for pointing out this error.
In the last paragraph on p. 29, lines 2 and 3, the "b" in pKb should be in a smaller font.
On the second line of p. 35, the second formula should read PhCH=N+(O–)Ph. Thanks to Thomas Pillow for spotting this error.
In the penultimate sentence of the first full paragraph on p. 35, the first resonance structure for isocyanides should read R-N̎=C: (the triple bond should be a double bond). Thanks to Lexie Moore for spotting this error.
The list of Lewis-acidic transition metal complexes in the small-font paragraph in Section 1.6.4 erroneously includes AlCl3, and it is even questionable whether CeCl3 and ZnCl2 belong in the list. Thanks to Michael Garrison for spotting this error.
In the last figure on p. 59, the leftmost O atom is missing its – sign. Thanks to Dr. David Brown for spotting this error.
In the first mechanism on p. 61, the starting material for the overall reaction (first line) is benzophenone, but the starting material for the mechanism (last two lines) is benzaldehyde. The starting material in the first line should be changed to benzaldehyde, and the product should be changed to the N-substituted benzaldimine. Thanks to Feng Ni for spotting this error.
An extra CO2Et group magically appears in the final mechanism on p. 61, second line, second structure. It then disappears in the next structure. The mechanism should read as follows:
Thanks to Guido Kramp for spotting this error.
The penultimate sentence of the first paragraph on p. 63 should begin, "(Whether an aldol product is syn or anti....)" Thanks to David Flanagan for spotting this error.
In the last paragraph beginning on p. 79, I point out that, after halogen–metal exchange between RLi and ArX to give RX and ArLi, an SN2 substitution reaction can ensue. I should have described the product of this reaction as an apparent nucleophilic aromatic substitution product. Thanks to Tadashi Okuyama for pointing out this error.
In the third line of the third paragraph on p. 89, the word "substituent" is misspelled. Thanks to Feng Ni for spotting this error.
In the second-to-last line on p. 93, the formula of oxalyl chloride is written incorrectly. It should be (COCl)2. Thanks to Yang Yang for spotting this error.
In the figure at the top of p. 95, the configurations of the alcohol and the two subsequent intermediates derived therefrom have been inverted. The correct figure is:
Thanks to Andrew Nelson for spotting this error.
In question gg, the product should be trans, not cis. Thanks to an unnamed reader for reporting this error.
In the figure at the bottom of p. 106, the third structure in the top row shows a C–H σ bond adjacent to the carbocation, but the caption next to the figure calls it a C–C σ bond, as does the orbital interaction diagram below it. The caption and diagram should both refer to C–H σ bonds. However, both C–H and C–C σ bonds stabilize neighboring carbocations, as do other σ bonds, most prominently C–Si σ bonds. In fact, C(sp3)–C(sp3) σ bonds are slightly better at stabilizing neighboring carbocations than C(sp3)–H bonds are because the former are slightly higher in energy.
Thanks to Matthias Emanuel for spotting this error.
In the fourth and fifth lines on p. 129, +N≡O should be changed to N≡O+. Thanks to Michael Garrison for spotting this error. Also, in the third line on the same page, there should be a space between the period and the subsequent word.
The third sentence of the first full paragraph on p. 133 should read, "Protonation of the carbonyl O occurs ...." Thanks to Benjamin Pollock for spotting this error.
Formaldehyde is probably insufficiently basic (pKa ca. –5) to be protonated by a simple ammonium salt (pKa ca. 10). In the absence of a slight excess of free acid, the reaction of a ketone or aldehyde with an ammonium salt to give an iminium ion probably proceeds by the basic mechanism, p. 61, with solvent acting to deprotonate the ammonium ion initially. Thanks to Prof. Edwin Vedejs for pointing out this error.
In the second and third full sentences on p. 156, ψ3 and ψ4 should be changed to ψ2 and ψ3. Thanks to David Weber for spotting this error.
In the first paragraph on p. 159, the conversion of the cyclopropylidene to the allene should be described as a four-electron electrocyclic ring opening. Thanks to Kaiyue Zhao for spotting this error.
In the second paragraph on p. 160, "diphenylacetic acid" should be changed to "methyl phenylacetate". Thanks to David Weber for spotting this error.
The third structure in the mechanism towards the bottom of p. 177 contains a pentavalent N atom. The +N=O should be +N–O–. Thanks to James Kirkham for spotting this error.
About 1/4 of the way down p. 184, between the dashes, the ortho–para rule is stated incorrectly. It should read, 'the most electron donating substituent on the diene and the most electron withdrawing substiuent on the dienophile are "ortho" or "para" in the product....' Thanks to Yiquan Zhao for pointing out this error.
In the third paragraph on p. 186, the two out groups on the dipole (not the dipolarophile) become cis in the product, as do the two in groups. Thanks to Tadashi Okuyama for pointing out this error.
In the paragraph on p. 189 just above the first graphic, the first sentence should end with, "..., a very electron rich alkene is allowed to react with a very electron poor alkene." Thanks to Matthew Duffy for pointing out this error.
In the fifth paragraph on p. 199, the substrate for the Wittig rearrangement is best described as an allyloxycarbanion. Thanks to Tadashi Okuyama for pointing out this error.
In the figure at the top of p. 200, the ether side chain in the starting material should have α stereochemistry, not β as shown. Thanks to Razi Hussaini for pointing out this error.
The substrate in the figure at the top of the page has (S,E) stereochemistry, not (R,E) as the text says. Thanks to Michael Mandler for pointing out this error.
In the last line of graphics on p. 208, the OH group should be pseudoaxial, not pseudoequatorial, in the second structure, as shown:
Thanks to Matthew Campbell for spotting this error.
all-cis-1,3,5,7-Cyclononatetraene can theoretically undergo four different electrocyclic ring closures, not just three.
Although I have not seen single-electron curved arrows (fishhooks) used to indicate a one-electron transfer reaction, Prof. Edwin Vedejs tells me that he has seen it, so feel free to use it.
At the end of the first paragraph on p. 244, the text says:
The word "Initiation:" should follow the first line of structures in the Example on p. 247. Thanks to Hui Wang for spotting this error.
In the last paragraph on p. 253, the radical produced in the Barton reaction is best described as an alkoxyl radical. Thanks to Tadashi Okuyama for pointing out this error.
In the figure that shows an example of conjugate reduction, in the step where the dianion reacts with H–OEt, the electron-flow arrow that starts at the H–O bond and points to O should be a two-electron arrow, not a one-electron arrow as shown.
In both Examples on p. 259, the reagents above the arrow on the first line of graphics should read "4 Na", not "2 Na". Thanks to Matthew Campbell for spotting this error.
The second sentence on p. 272 should read, "Transition metals, by contrast, have nine valence AOs ...." Thanks to Hui Wang for spotting this error.
In the Sharpless dihydroxylation and aminohydroxylation examples on p. 294, the (2R,3S) isomer would be produced by the DHQ-based ligand, and the (2S,3R) isomer would be produced by the DHQD-based ligand. Thanks to Marius Aursnes for spotting this error.
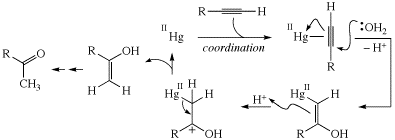
In the catalytic cycle for alkyne hydration on p. 296, in the upper right structure, the two arrows should lead to and away from the opposite C atoms in the alkyne.
Thanks to Jeffrey Johnston for spotting this error.
The problem should read:
In the first line of the last figure on p. 304, the number of CO ligands attached to Cr should decrease from four to three in step (a). Thanks to Yiquan Zhao for pointing out this error.
In the first full paragraph on p. 317, the product of the Sonogashira reaction should be described as an internal alkyne. Thanks to Tadashi Okuyama for pointing out this error.
The product is drawn incorrectly. The correct reaction is:
Thanks to Bo Yingjian for spotting this error.
The N atom in the product is missing an H atom.
Thanks to Yiquan Zhou for spotting this error.
The discussion of the Jacobsen epoxidation begins on p. 291, not p. 296 as listed in the index. Thanks to Sithamalli Chandramouli for spotting this error.
The index contains an entry for "Rhenium (Rh)". It should read "Rhodium (Rh)". Thanks to Ryan Michael for spotting this error.
The index says that the Shapiro reaction is discussed on pp. 85–87, but none of the reactions on these pages is the Shapiro reaction. Although the Shapiro reaction also involves the addition of an organolithium to an N-sulfonylhydrazone, it takes place under very cold conditions and does not generate a free carbene. Thanks to Prof. Edwin Vedejs for spotting this error.
In the index entry for triphenylmethyl, its abbreviation, trityl, is lacking the "r". Thanks to Theresa O'Sullivan for spotting this error.
In the answer key to Chapter 2, the discussion of problem 2.11 should refer to elimination–addition, not addition–elimination. Thanks to Dr. David Brown for spotting this error.
In the answer key to Chapter 4, the answer problem 4.2(g) should say that the CO2Me groups remain cis. Thanks to Michael Mandler for spotting this error. Also, the application of the out–endo–cis rule is not clear because the 1,3-dipole is linear; instead, remember that the Ar group and the CO2Me groups will form the more sterically congested TS, and hence they will be cis in the product.
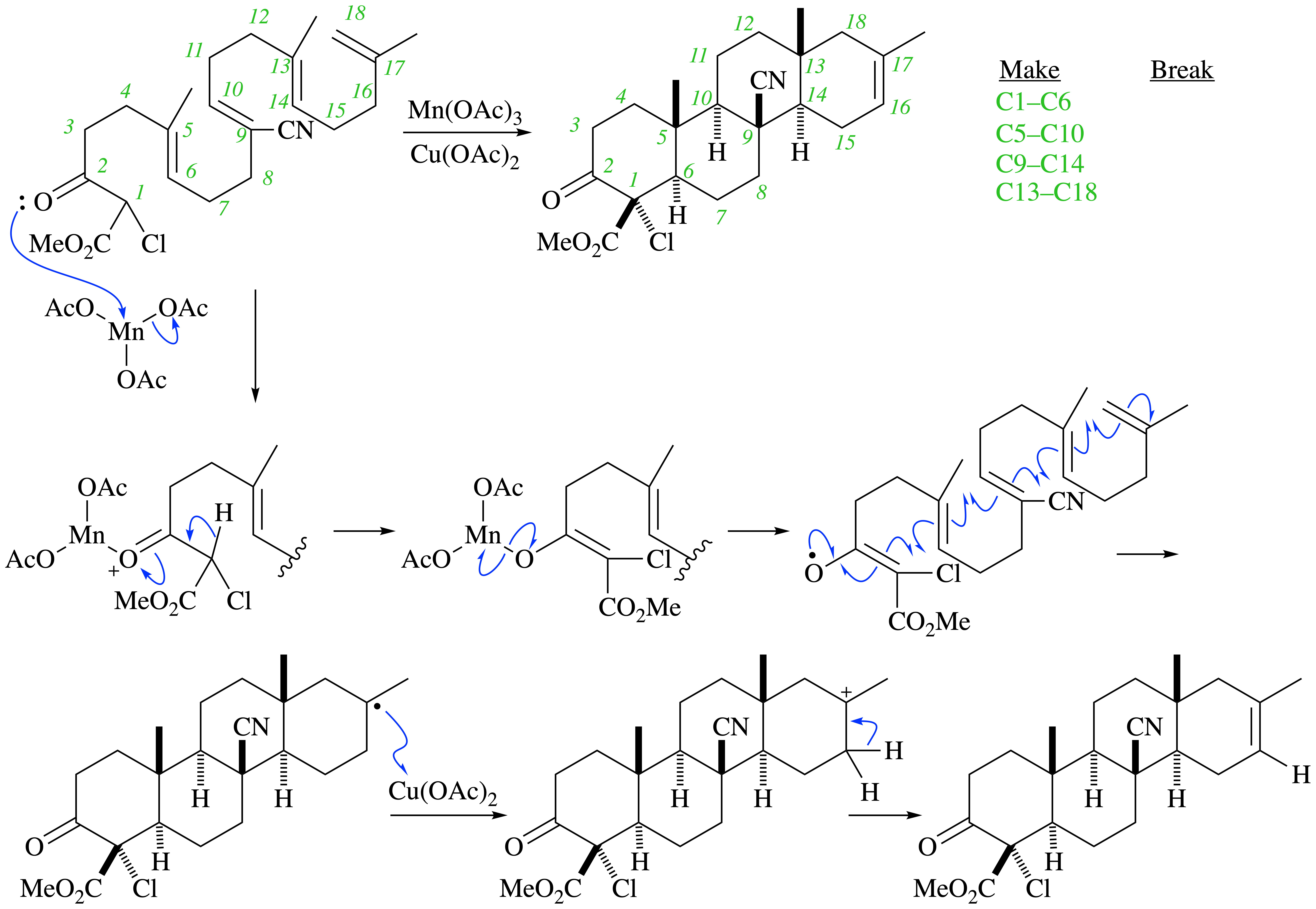
Two one-electron oxidations occur in this reaction. First, the β-keto ester forms a Mn(III) enolate, and the Mn–O bond undergoes homolytic cleavage to give Mn(II) and an O radical. A series of radical cyclizations takes place to generate a tetracyclic compound with a 3° alkyl radical. This compound transfers one electron to Cu(II) to give a carbocation, which undergoes C–H bond fragmentation to give the observed product.
Thanks to Maithili Pokle for reporting this omission.
Inconsistent solvent for nucleophilicities (pp. 29 and 57)
Problem numbering (pp. 24 and 38)
Terminology error (p. 22)
Font size error (p. 29)
Nitrone structure (p. 35)
Isocyanide resonance structures (p. 35)
Lewis-acidic transition metal complexes (p. 42)
Missing – sign on enolate O (p. 59)
Wrong starting material in imine mechanism (p. 61)
Extra CO2Et group in enamine mechanism (p. 61)
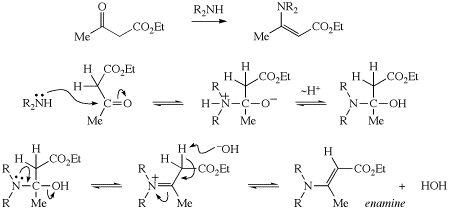
Syn or anti aldols (p. 63)
Substitution after halogen–metal exchange (p. 79)
"Substituent" misspelled (p. 89)
Formula of oxalyl chloride (p. 93)
Mitsunobu reaction mechanism (p. 95)
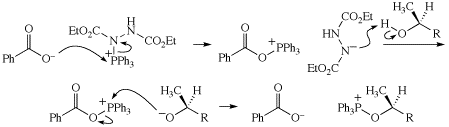
Stereochemistry of product (p. 103)
Carbocation stabilization figure (p. 106)
N≡O+ (p. 129)
Protonation of carbonyl group (p. 133)
Iminium ion formation under acidic conditions (p. 138)
Orbitals with zero coefficients (p. 156)
Cyclopropylidene to allene reaction (p. 159)
Compound name (p. 160)
Pentavalent N in nitrile oxide mechanism (p. 177)
Ortho-para rule (p. 184)
Dipole stereochemistry (p. 186)
Third type of [2 + 2] cycloaddition (p. 189)
Nomenclature error (p. 199)
Stereochemistry of Wittig rearrangement substrate (p. 200)
Stereochemistry of Claisen rearrangement substrate (p. 207)
Oxy-Cope rearrangement (p. 208)
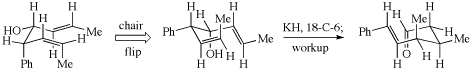
Cyclononatetraene cyclizations (p. 217)
Using single-electron arrows to show electron transfer (p. 237)
Regiochemistry of addition under polar vs. free-radical conditions (p. 244)
The anti-Markovnikov regiochemistry derives from the addition of the Br· radical to the less substituted C of the alkene (steric reasons) to give the lower energy, more substituted radical (electronic reasons). In a polar reaction, Br· would add to the more substituted C of the alkene.
The second sentence is inaccurate, because there is no Br· produced under polar conditions. It is better to say:
The anti-Markovnikov regiochemistry derives from the addition of the Br· radical to the less substituted C of the alkene (steric reasons) to give the lower energy, more substituted radical (electronic reasons). The eventual product has the Br atom attached to the C atom that was less substituted in the original alkene. By contrast, in a polar reaction, the Br atom ends up attached to the C atom that was more substituted in the original alkene.
Thanks to Yiquan Zhao for pointing out this inaccuracy.
Initiation (p. 247)
Nomenclature error (p. 253)
Wrong electron-flow arrow used (p. 255)
Acyloin condensations (p. 259)
Number of valence atomic orbitals (p. 272)
Sharpless dihydroxylation (p. 294)
Alkyne hydration mechanism (p. 296)
Problem 6.13 (Section 6.2.10, p. 303)
NMO oxidizes one CO ligand of the alkyne-Co2(CO)6 complex to CO2 and gives an alkyne-Co2(CO)5 complex. Write a mechanism for this transformation.
Migratory insertion of CO (p. 304)
Sonogashira product (p. 317)
Problem 6.28 (p. 325)
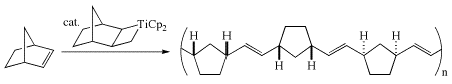
Problem 3(f) (p. 332)
Index entry for Jacobsen epoxidation (p. 348)
Index entry for Rh (p. 352)
Shapiro reaction (p. 352)
Triphenylmethyl abbreviation (p. 354)
Answer to problem 2.11 (Chapter 2 answer key, p. 7)
Answer to problem 4.2(g) (Chapter 4 answer key, p. 14)
Omitted answer (Chap. 5 answer key, end-of-chapter question 3ee)