PLS
622: Plant Physiology I, September 15, 2006
Section II: Embryo and Seed
Development:
Lecture
X: Seed
Dormancy:
IMPORTANT
TOPICS TO BE COVERED:
Seed
dormancy:
We will learn about...
- why dormancy is a sound strategy in
some instances.
- how the plant hormone
- that the metabolism of the dormant
seed can be different from that of a seed that has completed germination:
- about the role phytochrome and the
quality of light impacting the seed has on the dormancy status of some seeds.
We will also investigate how two different phytochromes can influence the
dormancy status of arabidopsis seeds.
- We will briefly go over skotodormancy
(a.k.a. secondary dormancy).
Seed dormancy:
First, dormancy is not a trait that is
unique to seeds. Buds too can be dormant in plants that grow in climates that
have seasonal variations in favorable growing conditions. These buds can be on
tubers and bulbs, or be the apical meristems of shoots and roots. In a larger
context, dormancy is also a trait of many life forms spanning diverse kingdoms,
Monera, Protista, Fungi, Plantae, even Animalia.
Quiescent vs dormant: Live seeds in
which none of the germination events are taking place, usually due to a low
moisture content, are said to be quiescent. They are alive and have metabolism
ongoing at a barely detectable rate but some environmental factor necessary for
germination to commence (usually the addition of water) is lacking. Seeds that
are in an environment optimal for germination, that is to say they are provided
with ample water, heat, light, and oxygen and yet fail to complete germination
are said to be dormant. The block to the completion of germination is an
attribute of the seed itself and must be removed before the seed will be able
to complete germination under favorable conditions.
Why
dormancy?:
Because the purpose of the seed is to complete germination and produce the next
generation of plant why some seeds should be shed with an inherent block to
germination is not immediately obvious. It can be argued that the developing
embryo of all seeds goes though a period of dormancy because, upon removal from
the seed, slight desiccation, and germination on nutrient media, many embryos
can complete germination whereas they cannot do so in the seed. This inability
to precociously complete germination has been found to be largely due to
elevated
The
role of
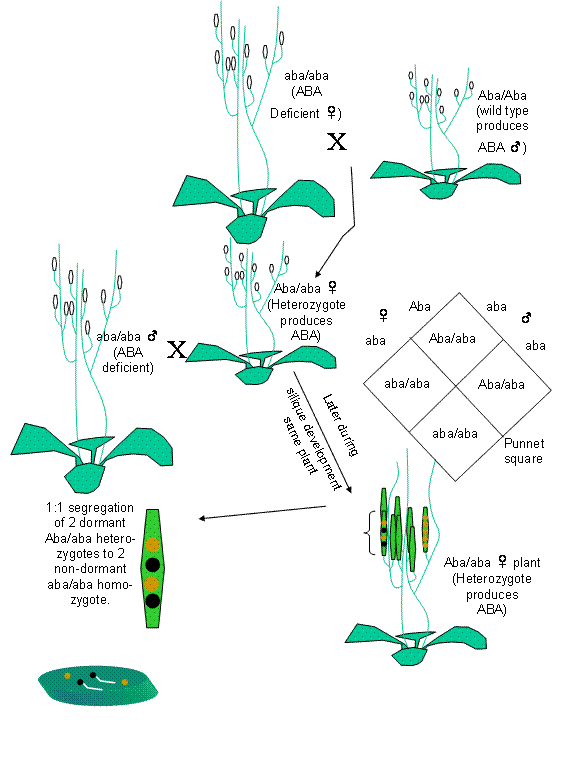
Figure 1: Seed dormancy in
arabidopsis is installed by seed produced
Metabolism
of dormant seeds: There have been many different metabolic pathways
hypothesized to play a role in alleviating dormancy in seeds over the years
that this phenomenon has been studied. The synthesis of nucleic acids or
proteins has been implicated as has a switch in metabolism which alters gas
exchange, adenylate charge and/or abundance or uses an alternative pathway to
produce energy and reducing power such as the pentose phosphate pathway (PPP),
reverse glycolysis and cyanide-insensitive pathway. Some of the evidence
accumulated has debunked several of these metabolic changes from having any
influence on dormancy alleviation. Others, such as the PPP, are still under
investigation. Support for the PPP being involved in dormancy alleviation comes
from observations that the dormancy of some species seeds can be alleviated by
the application of inhibitors of respiration. Substances that inhibit terminal
oxidation and the tricarboxylic acid pathway are effective in alleviating
dormancy in these seeds as are inhibitors of glycolysis. Additionally, electron
acceptors can alleviate dormancy. These inhibitors are thought to function by
diverting cellular oxygen from regular respiration to the PPP, where it is used
to oxidize NADPH to NADP. The electron acceptors replace oxygen in this
capacity and again result in elevated amounts of NADP. However, studies have
shown that the NADP/NADPH ratios are completely unrelated to the dormancy
status of the seeds. Whatever is occurring to alleviate dormancy upon
application of inhibitors of respiration or electron acceptors, it does not
appear to be due to elevated NADP amounts. On the other hand, Bob Buchanan and
co-workers are continuing to elucidate the role thioredoxin plays in permitting
the reduction of disulfide bonds in storage proteins and inhibitors of
alpha-amylase thereby permitting storage protein utilization in the first
instance and inhibitor inactivation in the second. Thus, the PPP pathway, the
only known source of NADP/NADPH in the seed, is the sole source of reductant to
reduce thioredoxin, via the enzyme NADPH thioredoxin reductase, back to a form
capable of reducing disulfide bridges since NAD/NADH is not capable of this
reaction (Fig. 2).
One of the problems defining metabolic
limitations that impose seed dormancy is that different species seeds behave
differently and may alleviate dormancy through a different metabolic switch
than others. Another is accurately determining the dormancy imposing tissue.
For instance, lettuce embryos appear to be constrained by the endosperm and it
is this tissue that imposes dormancy upon them in the absence of light.
Illuminate the seed and you alleviate dormancy. This could occur through either
cell wall weakening of the endosperm or
increased embryo thrust permitting them
to push through the endosperm opposing their expansion. Excised embryos
complete germination in darkness with no apparent dormancy. However, if they
are placed under water stress, the excised embryos germinated in the dark fail
to elongate at much less sever water deficits than embryos germinated in the
light suggesting that light enables the lettuce embryo to generate more thrust
than is possible in the dark. So, the embryos in light have a lower water
potential than embryos germinated in darkness. What is different between the
two? Are the light-germinated-embryos more osmotically active or do they have
more extendable cell walls resulting in lower turgor pressure? Measurements of
osmotic potential failed to reveal differences between light-germinated and dark-germinated
embryos. However, a decrease in turgor pressure has been documented in response
to light. This means that the cell walls of the embryo, when it is illuminated,
weaken, allowing the cells to elongate more easily than if the embryo was held
in darkness. The mechanism though which light acts appears to be in a pH
decrease in the apoplast. There is a vacuolar proton ATPase that is upregulated
during germination in tomato, but to date, no report of any of the number of
plasmamembrane proton ATPases known to exist being likewise regulated has been
documented. However, isolated lettuce embryos do seem capable of decreasing the
pH of the media they are in if illuminated, providing strong evidence that such
a proton pump does exist.
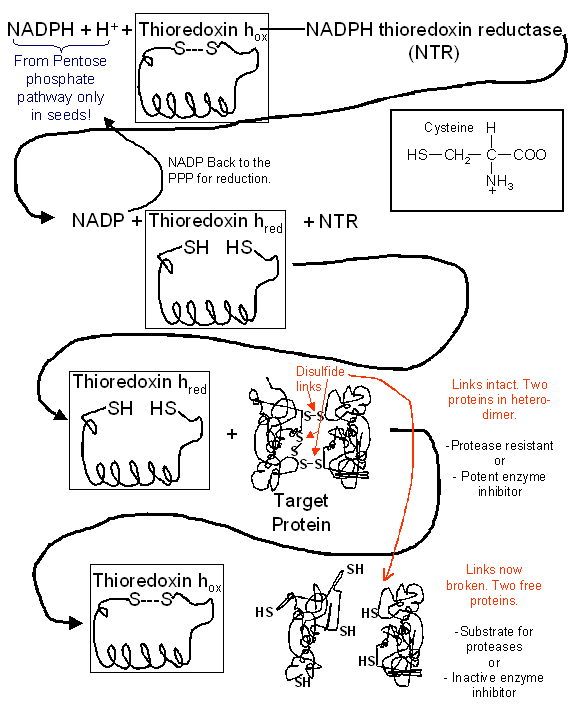
Figure 2: The necessity of the Pentose phosphate pathway to provide a source of NADPH to the cells of the germinating seed. Thioredoxin h activity permitting catabolic utilization of stored reserves depends on the recycling of thioredoxin h to the reduced form via NADPH.
Phytochrome
and Dormancy: One of the more spectacular discoveries in plant physiology
involved the control light quality has on the ability of many species seeds to
complete germination. There are no fewer than 5 phytochromes present in
arabidopsis. These chromophores were first discovered and investigated due to
the marked effect phytochrome B and possibly others, has on lettuce seed
germination. When imbibed seeds of lettuce were illuminated with a period of
far-red light, the percentage of seeds that subsequently completed germination
in the dark was very low. If the period of far-red illumination was followed by
a period of red light illumination, the seeds subsequently completed
germination to almost 100%. If however, the second, red light illumination was
followed by another period of far-red illumination, seed germination was again
drastically inhibited in the dark. This cycle of germination inhibition and
stimulation can continue ad infinitum
until, at some point much advanced in seed germination, the seeds “escape” from
phytochrome control and complete germination in the dark regardless of the
illumination they perceived last.
The
antagonistic nature of red/far red illumination on seed dormancy has led to
intensive
investigation of phytochrome states after
illumination from light of different spectral qualities.
As
mentioned previously, there are at least 5 different phytochromes present in
arabidopsis. Of the phytochromes, A and B are by far the best studied, having
been cloned and for which null mutants are available. Studies of the
phytochrome A deficient, phytochrome B deficient, and double mutant, have led
to the conclusion that both phytochrome A and B play a role in seed
germination. Phytochrome A, upon absorbing far red light is actually stimulatory
for germination while phytochrome B is the phytochrome that is purportedly
responsible for the photoreversible effect on seed dormancy. Recently, however,
the arabidopsis double phytochrome AB null mutant was used to show that seed
germination is still under phytochrome control, signifying that at least a
third phytochrome is involved in determining light regulated seed dormancy in
arabidopsis.
Studies with
microbeams of intense light at different wavelengths has demonstrated that
phytochrome is present in greatest amounts in the axis of embryos and very
little is present in the cotyledons. This is significant because it is the
elongation of the radicle that leads to the completion of germination and the
phytochrome control of dormancy probably should be localized to this portion of
the embryo rather than the cotyledons. No mention was made of whether
phytochrome was also found in the endosperm. This is not crucial to our
interpretation below that, in some seeds at least, phytochrome mediated endosperm
wall weakening might control dormancy and germination. It is fully possible
that, even if phytochrome is present only in the axis, its photoconversion to
the active form Pfr elicits a response from the radicle cells that includes the
production and transport of a signal to the cells of the endosperm in the
immediate proximity of the radicle i.e. the micropylar endosperm. This signal
would then dictate that the endosperm cells should produce enzymes that would
weaken their cell walls.
Partial cell wall disassembly and
dormancy: Recently more interest has been directed to determining whether or
not the cell walls of the endosperm cap region in endospermic seeds weakens
during dormancy alleviation. If the micropylar endosperm does not weaken, as we
saw in the gib-1 mutant of tomato and
ga mutant in arabidopsis, the embryo
will not complete germination. There does appear to be some evidence suggesting
that endosperm weakening does have to occur in several species and that this
weakening might be prevented in seeds that are dormant. The enzymes that have
been investigated to date all hydrolyze hemicellulose present in the cell walls
of the micropylar endosperm. In Datura
ferrox endo-b-mannanase and
a cellulase, both of whose transcription is under phytochrome control,
accumulate prior to radicle protrusion and endosperm cap weakening. In lettuce,
endo-b-mannanase
also appears to be under phytochrome control and may accumulate prior to
radicle protrusion, although this is contentious. In tomato several forms of endo-b-mannanase
exist, one of which is apparently specific to the endosperm cap the gene of
which is downregulated by
Secondary
(skoto) dormancy: Secondary dormancy is brought about when fully hydrated,
mature seeds experience gravely sub-optimal germination conditions. Some of the
conditions that invoke secondary dormancy include, anoxia, darkness,
excessively strong light, higher (lower) than maximal (minimal) temperatures
for the completion of germination, and water stress. These conditions do not
universally elicit secondary dormancy for all species, some species are
affected and other are not. Whatever the mechanism whereby skotodormancy is
initiated,