PLS 622, Plant
Physiology I: Friday, September 22, 2006
Vegetative
development: Trichome, root hair, and stomatal Development:
Objectives:
- To examine the
development of three specialized epidermal cell types, trichomes, stomata, and
root hairs.
- To explore our
knowledge of how the molecular control of trichome spacing is thought to occur.
- To construct an
overview of the roles of the similar molecular players in the various
developmental processes mentioned above.
Epidermal cell fate: Hitherto we have discussed cellular
differentiation in the context of the maturation, from a ground meristem, of a contiguous
array of cells comprising a particular tissue type. Assemblages of epidermal
cells provide an interesting contrast in that there are two types of highly
specialized cells present throughout the epidermis that develop in isolation,
surrounded by pavement cells. These are the guard cells (making up the stomata), and the trichomes/root hairs. Two
fundamental questions can be asked 1) how are the cells destined to
differentiate into guard cells or trichomes/root hairs selected? 2) How is the
spatial patterning of stomata and trichome/root hair placement in the pavement
controlled?
Trichome Development:
Trichomes are present on the aerial portions of almost every
terrestrial plant. Although some plants have but a single type of trichome, trichomes
exist in several different forms, often on the same plant. Tomato has six
different types of trichome. Trichomes are thought to perform a variety of
functions, from plant defense (physical obstruction to marauding insects, to
sites of synthesis and display of complex chemical inhibitors and repellents)
to creation of a boundary layer of moister air to reduce transpirational loss
of water. The nucleus of the cell resides in the base of the trichome and a
thin strand of cytoplasm is continuous into the cell extension which is highly
vacuolate. The cell wall of the mature trichome is thick and covered with
numerous papillae the function of which is unknown. The most impressive of trichomes
is that of the cotton ovule. The single cells of the ovule are capable of
supporting a rate of cell extension (trichome elongation) in excess of 2 mm a
day, leading to a final single cell length of 30-35 mm!!!
Trichomes mature basipetally. They expand laterally as the
cell extension elongates so that the girth as well as the length of a mature trichome
is much greater than that of the undifferentiated trichoblast. Additionally, a
rosette of normal atrichoblast epidermal cells form a group of support cells at
the base of the trichome stalk. Mature trichomes also increase their DNA
content per cell above the usual diploid content by a process known as
endoreduplication (synthesis of additional copies of the genome without
subsequent cell division). This may be due to the fact that the trichome,
although comprised of a single cell or a few large cells, expands considerably
via the cell extension and will therefore require additional copies of genes
for house-keeping enzymes if the cell is to remain efficient. It seems logical
that the cellular volume and the surface area serviceable by a single nucleus is
limited and strictly correlated with the C-value (number of haploid genomes) present
in a nucleus.
Trichome spacing.
So, how is trichome spacing determined? There are two
models. The first suggests that precursor cells produce inhibitory signals that
prevent other, neighboring cells, from going down the same developmental pathway.
The second suggests that, once a precursor cell is induced to become a trichome
it undergoes several divisions that separate it from other trichomes by a group
of cells all derived from the same precursor daughter cell. In fact the first
hypothesis appears to most closely describe control of trichome patterning. Let
us see how the second hypothesis was debunked.
The second hypothesis has been refuted by cell lineage
experiments using a transposon interrupted beta-glucuronidase (GUS) gene. With
the transposon in place the gene does not make a functional enzyme and cells expressing
the interrupted GUS gene stay clear in the presence of substrate. In cells that
have had a transposition event occur, the excision of the transposon leads to
the production of functional GUS from the now transposon-free gene and cells in
which this occurs can turn the substrate blue. The transposition event can be
induced in some cells prior to their mitoses in the expanding epidermis and
thereafter, all daughter cells produced from the affected cell will be able to generate
a blue color from the substrate. It was determined that the boundary of the
cells developing from such transposition-positive cells passed through trichome
producing and non-producing cells randomly, debunking the second hypothesis
(Fig. 1).
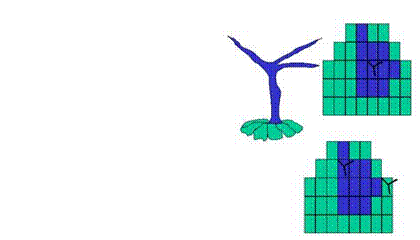
Figure 1: Trichome cell
lineage experiments using a reporter gene.
Another way in which trichome
spacing may be maintained is that the precursor cell, undergoes asymetric
division with the different sized cells resulting in different developmental
fates. Although this does occur in some plants (e.g. many monocots) it is not a
universal method of determining trichome spacing. Additionally, epidermal cells
of
arabidopsis can be artificially induced to form trichomes
without the requirement of undergoing cell division.
There are at least 10 known genes
that affect trichome development, of which we shall discuss 7. There are three
core protein types in trichoblasts (cells destined to become trichomes) and
three in atrichoblasts. Two of these core protein types are the same in the two
types of cells. The trichoblast stimulating complex is formed from GL1:GL3:TTG1
(GL1 = GLABRA1; GL3 = GLABRA3 and; TTG1 = TRANSPARENT TESTA GLABRA1). The
atrichoblast complex is formed from TRY:GL3:TTG1 (TRY = TRIPTYCHON). It is the
competition between TRY and GL1 to interact with the other proteins in the
complex that decides trichoblast fate. The GLABRA1
(GL1) gene (glabrous = "free
from hair") which, when defective (gl1),
results in the loss of trichomes on most surfaces, and the TRY gene, are both
MYB transcription factors (The MYB
moniker is from the first protein of this type identified, an oncogene from the
Avian Myeloblastosis virus, a DNA binding
protein). The GLABRA3 (gl3) protein and ENHANCER OF GL3 (EGL3)
protein both encode basic Helix-Loop-Helix (bHLH) proteins that interact as
homo (GL3:GL3 or EGL3:EGL3) or hetero-dimers (GL3:EGL3) and act as scaffolding
to which TTG1 and GL1/TRY proteins bind. Neither the gl3 nor the egl3 mutants
have striking phenotypes but the double mutant is totally without trichomes. The
TRANSPARENT TESTA-GLABRA1 protein is a member of both the trichoblast
simulating and trichoblast inhibiting complex. Mutants in ttg1, as its name suggests, is a pleiotropic mutant that results,
among other things, in the loss of trichomes from most surfaces of the plant,
akin to gl1 mutations. An additional
player that influence this competition is CAPRICE (CPC), also a MYB
transcription factor (a DNA binding protein). CPC negatively regulates trichome
initiation by binding to GL1, making it unavailable to participate in the
trichome initiating complex. Both the trichoblast stimulating and the
trichoblast inhibiting complex bind to the promoter region of the GLABRA2 (GL2) gene (encoding a homeobox protein). In trichomes, GL2 is up-regulated by the trichoblast
stimulating complex (GL1:GL3:TTG1) and down-regulated by the trichoblast
inhibiting complex (TRY:GL3:TTG1).
The model in figure 2 attempts to
synthesize what we know about the control over trichome initiation and spacing
in arabidopsis. Initially, all cells of the leaf have an equal opportunity to
become trichoblastic. However, early during development, one of the cells at
the margin of the distal portion of the leaf primordia acquires the
trichoblastic fate through a fortuitous stimulation of GL2. From now on,
trichoblast acquisition is dependent on how close the nearest trichoblast is to
an undifferentiated cell. The complexes (stimulating or inhibiting) require two
bHLH proteins to associate (GL3:GL3; EGL3:EGL3 or; GL3:EGL3) and they hold TTG1
(a WD-repeat protein [W = tryptophan; D = aspartic acid] sometimes called
WD40-repeat proteins because the conserved W and D of the motif is within a
region of approximately 40 amino acids) and either GL1 (a MYB protein) or by
TRY (another MYB transcription factor) in association. If the trimeric protein
complex GL1:GL3:TTG1 is formed, the cell is stimulated to adopt the trichome
fate (Fig. 2). If the trimeric protein complex TRY:GL3:TTG1 is formed, the cell
is steered into the atrichoblast fate. The CAPRICE protein CPC is a negative
regulator of trichome initiation in arabiodopsis due to its propensity to
sequester GL1 protein away from the initiation complex, providing more GL3 and TTG1
subunits for interaction in a trichome-limiting complex with TRY (Fig. 2).
The timing of trichome
development:
The timing of trichome development varies among plants. In
arabidopsis leaves, although the first trichome does not commence
differentiation, at the distal leaf tip, until the leaf primordium has expanded
to circa 100μm, the trichomes are the first cells to terminally
differentiate. On cotton ovules, the trichomes (which ultimately become single-celled
cotton fibers) do not commence elongation until after all other epidermal cells
have ceased to divide.
Fusing the nuclear targeted maize R gene that promotes
trichome formation, to a steroid receptor from mice permitted the inducible expression
of the R gene in the trichome-less transparent
testa glabra1 (ttg1) mutant. With
no steroid application, the R gene remained cytosolic and the mutant remained
trichome-less. Upon application of the steriod, the R gene product traversed
the nucleous and the mutant reverted to a trichomed phenotype. When application
of steroid was delayed, the more distal, older, sections of expanding leaves
failed to revert, signifying that the trichome precursor cells are determined
early in leaf expansion.
Additionally, the REDUCED
TRICHOME NUMBER (RTN) gene may be
responsible for determining the duration a particular cell is competent to make
trichomes. This gene has been shown to be an allelic variant between Landsberg
erecta and
There is also some evidence that TTG1 and/or GL1 may be
involved in determining the spacing of trichomes. Weak mutant alleles of both
TTG1 and GL1 have been identified that do not completely suppress trichome development.
In these plants, trichomes appear in clusters. Additionally, heterozygous TTG/ttg
plants that ectopically overexpress GL1 often develop numerous clusters of
trichomes. This suggests that a stoichiometric balance between TTG1 and GL1
must be maintained to prevent trichome clustering. An additional gene, TRY, may
act downstream of TTG1 and GL1. Mutations in this gene increases the numbers of
trichomes that occur in clusters.
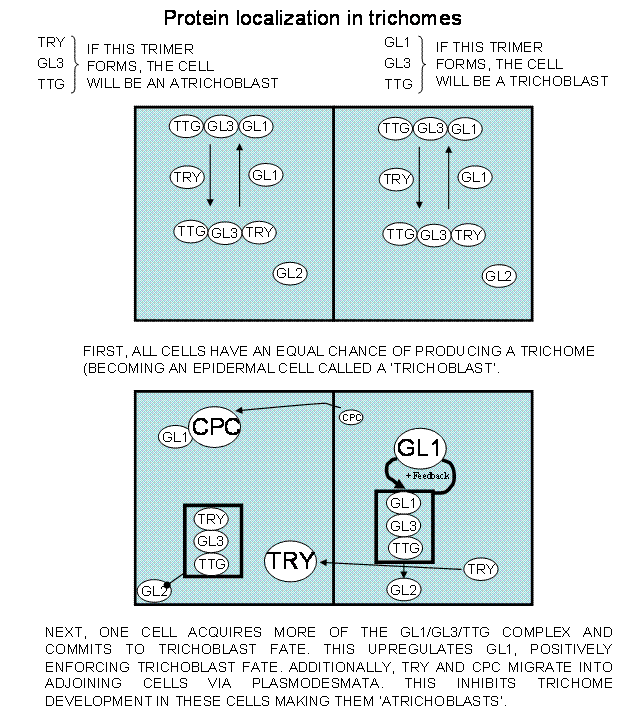
Figure 2: A Model of
trichome initiation/suppression.
References used in the preparation of these notes.
Costa S, Dolan L. 2003. Epidermal patterning genes are
active during embryogenesis in Arabidopsis. Development 130, 2893-2901.
Koshino-Kimura Y, Wada T, Tachibana T, Tsugeki R, Ishiguro S
and Okada K. 2005.
Regulation of CAPRICE Transcription by MYB Proteins for Root
Epidermis
Differentiation in Arabidopsis. Plant Cell Physiol. 46: 817–826.
Nadeau JA, Sack FD. 2002. Control of stomatal distribution
on the Arabidopsis leaf surface. Science. 296: 1697-1700.