PLS 622: Plant Physiology I, Wednesday, September 27, 2006.
Vegetative
development: Vascular tissue differentiation:
Just
prior to the completion of seed germination, the provascular tissue in the
embryo differentiates into protoxylem and protophloem, providing the rudiments
of a vascular system until more permanent metaxylem and metaphloem
differentiate. Thereafter, the shoot and root apical meristems produce
undifferentiated cells, some of which take on the vascular cell fate as the
plant body becomes larger and more complex. In some plants radial growth occurs
through the production of xylem, phloem and attendant tissues by a vascular
cambium (a secondary meristem). This vascular system pervades the plant body
providing the means of short and long range bulk transport of liquid and
dissolved solutes. In this lecture, we will learn some of the salient points
known about how the vasculature develops.
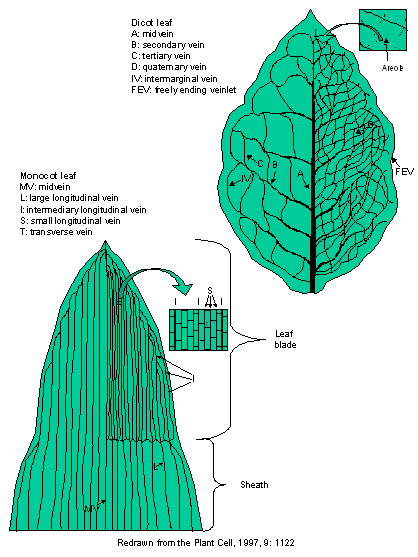
Pictured
below is a leaf from a dicot and a monocot showing, schematically, the typical
patterning of leaf venation. In monocots, the primary venation runs
longitudinally for nearly the length of the leaf. Monocot veins undergo
divergence at the base of the lamina and converge and fuse toward the leaf
apex. Their venation pattern is termed striate.
The venation of grass leaves is used as an example of vascular patterning in
monocots. For both dicots and monocots, veins of several different sizes (orders)
comprise the vasculature and, at the level of the smallest sized veins,
venation acquires an essentially reticulate pattern (forming areoles). Veins of
both dicots and monocots anastomose (join together) at the margins of the leaf,
with secondary veins connecting to other secondary veins at first and
eventually joining with the midvein(s). Although there are typically fewer vein
sizes in the monocots than there are in the dicots, as many as 6 have been
identified in both. All veins diminish in size through the vein orders as they
approach the leaf apex.
Vascular
pattern ontogeny:
Provascular
tissue and the ground meristem are both derived from the uniformly meristematic
tissue of the leaf primordium and only differentiate upon the commencement of
cell division and expansion associated with leaf development. It is therefore,
difficult to ascertain which cells are destined to become vascular tissue prior
to the commencement of their differentiation and hence, difficult to study
early events in vascular tissue ontogeny. One of the first events that occurs
to hint at a special developmental pathway for cells destined to become vascular
tissue is that they stain darker than surrounding non-vascular cells. This is
thought to be due to the greater vacuolization of the non-vascular cells at
this point during development. One interesting aspect of vascular tissue ontogeny,
regardless of the species in which it is studied, is that the cells do not
adhere to some of the rules of cell division in a growing plant part. One is
that there may be cell divisions longitudinally, perpendicular to the direction
of cell growth (formative division) increasing the numbers of cells in the
vascular strand.
Dicot:
The
vasculature of dicots develops through three major phases during leaf
morphogenisis and growth. First, the midvein provascular strand invades the
leaf acropetally from the stem provascular tissue into the leaf primordium.
Second, secondary provascular strands proliferate from the midvein and develop
in the leaf lamina towards the leaf margins. Finally, the provascular strands
of tertiary and higher veins are established during intercalary expansive
growth of these elements of the vasculature. In contrast with the acropetally
oriented development of the midvein, secondary veination develops in a species
dependant manner. In some, the secondary veins commence their outgrowth from
the midvein at the leaf apex and initiation from the midvein proceeds
sequentially down towards the leaf base (basipetal secondary vein development).
In others the opposite is true, with secondary veins arising from the midvein
in an acropetally oriented sequence. Finally, there are species that have
secondary leaf vasculature arise first in the mid-part of the leaf and
initiation proceeds both basi- and acro-petally. Development usually proceeds
basipetally for the higher orders of vasculature so that the minor veins are
present at the leaf apex while the secondary veins are still forming near the
petiole.
Monocot:
Most
grasses initiate the midvein and the secondary vasculature from the disk of
leaf insertion and not from the existing stem provasculature. The midvein
elongates acropetally toward the leaf tip. Only after the commencement of
acropetal growth does the midvein also commence development toward the stem
vascular strand, forming the leaf trace. Large longitudinal veins parallel to
the midvein also form in this manner, arising from the disk of leaf
insertion on the stem, developing first
acropetally in the elongating leaf and then basipetally to the stem
vasculature. Next, the intermediate provascular strands initiate in the leaf
apex and development extends basipetally to connect to the existing major
longitudinal veins that have developed in the leaf. Only some of the
basipetally extending intermediary veins develop through the leaf sheath to
connect with the stem vascular bundle through a leaf trace. Finally, small
longitudinal veins develop commencing near the apex of the leaf and extending
basipetally to connect to the higher order veins at about the leaf sheath-leaf
junction. The transverse veins also develop starting near the apex of the leaf
and extending basipetally to provide the leaf with a reticulate network of
vasculature. The reader is cautioned that the rather novel developmental
sequence with the midvein and large longitudinal veins commencing development
in the disk of leaf attachment without any apparent connection with the
provascular trace of the stem may be just that....apparent. Our limitations
identifying provascular tissue prior to its advanced development may prevent
the recognition of an existing leaf trace until after the midvein in the disk
of leaf attachment has commenced differentiation.
Vein
spacing:
The
most obvious manifestation of uniform vein spacing is seen in the leaf blade of
grasses that have a constant longitudinal vein number per unit lateral blade width.
In the dicots, even if the polygonal shape of the ultimate leaf areoles are
remarkably diverse, the occurrence of branch points from veins and veinlets is
remarkably uniform. Constant branch points from veins

that are undergoing intercalary growth
is seemingly maintained by the initiation of provascular tissue between
existing branch points and the growth of the new vasculature by intercalary
growth into the areole.
Models
for the Regulation of Vascular Pattern Formation:
Any
hypothesis that attempts to describe vascular pattern formation must account
for three divergent phenomenon; 1) the acropetally oriented formation of major
veins in developing dicot leaves; 2) the formation of isolated, parallel
provascular tissue in expanding grass leaves and; 3) the simultaneous formation
of minor veins and transverse veinlets in both dicots and monocots over large
areas of the leaf. The two best models only imperfectly describe how vascular
patterning might arise.
Model
1: Canalization of signal flow:
All
cells start out being equivalent transporters of auxin, a hormone implicated in
the induction of vascular differentiation. Stochastically, some cells transport
more auxin, and this greater contact with auxin enhances their ability to
transport more of it, creating a positive feedback loop. The greater auxin flux
through these cells eventually induces them to become provascular cells and
drains surrounding cells of auxin, inhibiting them from also becoming
provascular tissue. Additionally, the auxin is passed basipetally to the next
cell in the file which now accrues its own auxin plus all the auxin from the
cell above it, converting it to provascular tissue. This hypothesis can account
for the type of vascular development seen in dicot leaves but cannot account
for how the provascular tissue in monocots appears to develop, nor the
simultaneous development of minor veins throughout a large section of the leaf.
Model
2: Diffusion-reaction prepattern:
This
hypothesis requires two components: 1) a localized, positive feedback loop that
stimulates the further production of any transient increase in the amount of an
otherwise, uniformly distributed substance, stimulatory to vascular tissue
development (also known as a ‘stimulatory morphogen’) and; 2) the production of
a rapidly diffusing, long-range inhibitory compound from the same site producing
the stimulatory morphogen that radiates out from that site, inhibiting the
initiation of vascular tissue in the vicinity of the developing vasculature
(‘inhibitory morphogen’). This hypothesis can account for the parallel, and
simultaneous, formation of longitudinal veins in monocot leaves. Additionally,
it can account for the intercalary growth of new veins between older veins as
the leaf blade expands because the concentration of the inhibitory morphogen
would be depleted the further apart the two veins moved until it was no longer
sufficient to inhibit a new wave of provascular tissue formation. This would
also tend to promote very uniform spacing between veins, their simultaneous
formation, and produce patches where no venation would occur…areoles.
Current
theory is that, in order to explain much of what we know about vascular tissue
differentiation, we will have to come up with a joining of aspects of the two
models above.
Comparison
among leaf, stem, and root vasculature:
The
most noticeable difference between the vasculature of the root, stem and leaf
is in the symmetry of the organs. In roots, the vasculature forms a central
pith-filled, or solid cylinder that is radially symmetrical and whose
organization is not greatly influenced by the occurrence of peripheral organs.
In the stem, the vasculature is organized into radially symmetrical, sympodial
bundles whose organization is in direct relation to shoot phyllotaxis (i.e.
it’s organization is dependent on the attachment of the leaves to the stem
because an amount of the vascular trace must branch off from that of the stem
and enter each leaf to supply it with water and nutrients while removing
photosynthate). At each dicot node, at least three vascular bundles diverge
from separate sympodial bundles to serve the leaf at that node. The remainder
of the sympodial bundle continues through the next internode. The divergent
vascular bundles, so called leaf traces, arising as they do from independent
sympodial bundles, provide redundancy in the water supply of the leaf. The
architecture of the sympodial bundle seldom varies having the phloem situated
to the outside of the xylem. The position of the xylem towards the adaxial
(upper) portion of the typically dorsiventral leaf and the phloem towards the
abaxial region reflects the architecture in the stem from whence the leaf trace
originates.
Vegetative
development: Phloem and Xylem:
Let
us examine two components of plant vasculature, phloem and xylem.
One
fundamental difference in how animals and plants transport assimilate is that while both use vessels made from cells, the
smallest of these vessels in animals is comprised of cells but does not have
majority of the transport passing through the cells themselves but rather
through vessels formed by these cells capillaries. In plants of course,
transport is through the phloem and xylem cells themselves.
Vascular
differentiation in plants is difficult to study due to the position of the
vascular elements, buried within the plant body, the relatively few cells
comprising the vasculature, and the even fewer cells undergoing differentiation
at any one time relative to the number of differentiated vascular cells. Much of
what is known about vascular tissue differentiation at the molecular level has
been acquired in the past two decades with the advent of an inducible cell
culture system (Zinnia elegans) for xylem providing quantities of more-or-less
synchronized cells following the same developmental pathway. Tissue culture
systems have similarly been adopted for studies of phloem differentiation.
Development of ‘axial system’ (stem)
Xylem and Phloem:
In
the seedling there develops primary vasculature comprised of protophloem and protoxylem which are quickly crushed and torn apart as the seedling
elongates. They serve to transport water and nutrients during the early stages
of establishment and are quickly replaced by the metaphloem and metaxylem. This
vasculature is more long lived, developing after most of the cells comprising
the seedling have finished elongating. Additional files of cells are added to
the existing metaphloem and metaxylem as the meristems produce them.
While
the protophloem has no companion
cells, the metaphloem does, enabling
it to survive for considerably longer periods. Companion cells are associated
with mature sieve elements and are thought to be necessary for sieve element
function and survival. The role of the companion cell in phloem loading (sieve
element function) will be dealt with below. Correlative evidence supporting the
conjecture that companion cells are responsible for sieve element survival
arises from studies of protophloem
elements in developing leaves and stems and which lack companion cells. These
protophloem elements are short-lived after they have differentiated and are
replaced later in development by metaphloem
sieve elements which have companion cells and which live much longer (years in
the case of palms). The companion cells must produce the proteins for the
mature sieve elements they serve because the mature elements are without
ribosomes. Without a mechanism for producing proteins de novo the life span of any cell would be short indeed. Even the
P-protein, necessary for avoiding catastrophic failure and possible infection
of large portions of the phloem system and surrounding tissue upon injury of an
element, is manufactured in the companion cells and transported to the mature
element. This hypothesis has been demonstrated using a combination of in situ localization of P-protein mRNA
and immunolocalization of P-protein itself. P-protein has been localized to
both mature elements and their associated companion cells while P-protein RNA
has been located solely in the companion cells. Additionally, the SUT1 sucrose
transporter located in the mature sieve element plasmamembrane although its RNA
is synthesized in the companion cell. Finally, phloem exudates obtained from aphid stylets or cut stems contain many
hundreds of small (< 25KDa) proteins (sieve
tube exudate proteins; STEPS) that continue to exude from the phloem for
considerable sample periods. This continuous supply of newly-synthesized (data
from labeling studies) proteins strongly implicates the companion cells as the
site of synthesis, and the transport of the proteins through the plasmodesmata
into the sieve tubes.
For
those plants with secondary growth, both secondary xylem and phloem develop
from the vascular cambium. Associated with secondary xylem are ray cells which,
unlike the axially arranged xylem and phloem, are arranged radially. These rays
can move metabolites laterally through the bole of a tree, storing substances
otherwise toxic to cells in the heartwood or outer bark which eventually die.
Phloem:
For
phloem transport to be effective, all large organelles are degraded during
development so as not to impede the flow of assimilate though the cell. Hence,
the nucleus, vacuole, Golgi bodies, rough endoplasmic reticulum, and ribosomes
are missing from mature phloem sieve elements. Additionally, the plasmodesmata
of the sieve element are enlarged between adjacent sieve elements (sieve pores) to enhance flow of
assimilate between elements. Despite the
paucity of organelles, sieve elements are not dead and maintain a functional
plasma membrane, continuous through the sieve pores, that is essential for the
job they do. Thus, a series of sieve elements are bounded by a single plasma
membrane forming a syncytium,
essentially a single compartment.
During phloem
development, the phloem mother cell
divides to produce a phloem cell precursor and the precursor to a companion cell. The housekeeping of the
mature sieve element will be done by the companion cell that assumes the
regulatory responsibilities for the neighboring, enucleate sieve element. In
some plants, there are numerous plasmodesmatal connections, serving as a
symplastic pathway, among the sieve element, its attendant companion cell, and
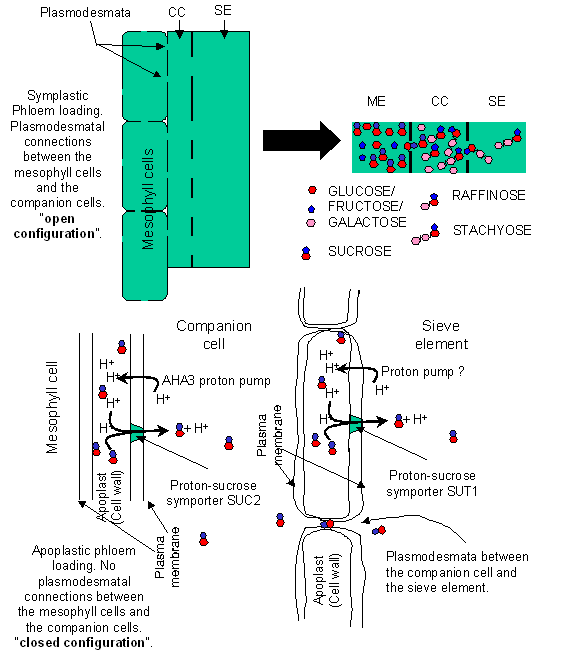
the mesophyll. In others the sieve element and its companion cell have a
paucity of plasmodesmatal connections with other cell types. This diversity has
provided support for the contention that phloem loading can occur in two
different methods depending on the species of plant. Plants are thought to load
material into the phloem via either: 1) symplastic-
or; 2) apoplastic-phloem loading.
The polymer-trapping hypothesis functions
in some species of plants and involves symplastic phloem loading. According to
this hypothesis, mono- and di-saccharides are small enough to be capable of
diffusing from mesophyll cells into companion cells along a concentration
gradient through plasmodesmata. In the companion cells these simple sugars are
combined into larger oligomers, oligomers of sufficient size to prevent their
diffusion back through the narrow plasmadesmata leading into the mesophyll
cells. However, due to the large diameter of the branch plasmadesmata leading
into the sieve element from the companion cell, these sugars can diffuse into
the sieve tube and be transported.
In contrast to
the symplastic route, some species have no plasmodesmatal connection between
the mesophyll cells and the companion cells. There is direct evidence for
phloem loading from an apoplastic pathway involving a proton pump AHA3, and a proton-sucrose
symporter SUC2, located in the companion cells. Sucrose, produced by the
mesophyll cells is dumped into the apoplast and then recovered into the
companion cells via SUC2. Regardless of which pathway is used, companion cells
are implicated in the delivery of material to the sieve elements. However,
recent evidence has led to the belief that a second apoplastic loading
mechanism exists in the sieve elements themselves. Immunolocalization
experiments have demonstrated the presence of a proton-sucrose symporter SUT1 in the plasma membrane of the phloem
sieve element.
Phloem translocation has been estimated
to be 40 cm/hr, and, due to the
proximity of the organelles to the flowing assimilate stream, it is possible
that the organelles are subjected to considerable shear forces. The organelles
must therefore, be anchored in place along the cell periphery. Additionally,
any intra-phloem transport of molecules not abundant in the translocation
stream must be compartmentalized, probably within the lumen of the sieve element
reticulum (SER).
Sieve elements
develop hydrostatic pressures in excess of 30
atmospheres! The cell walls of sieve elements are therefore, modified to be
able to contain this high pressure without bursting. One of the most
fundamental modifications is the production of cellulose microfibrils at right
angles to the axis of elongation of developing sieve elements. These
microfibrils act like hoops around a barrel, assisting the cell to maintain its
shape under the pressures developed within. Along with the obvious practical
advantage of not bursting, this reinforced cell will not undergo deformation
(bulging) although considerable pressure is applied within, thereby propagating
this pressure longitudinally along the phloem tissue.
Xylem:
Hormonal
control of xylogenesis:
Endogenous
auxin appears to be responsible for determining the initiation of tracheary element (TE) differentiation
and the size of the resulting TEs. Cytokinin, apart from enhancing the
sensitivity of tracheary initials to auxin, is also required for the induction
of TE differentiation and its progression to completion. There is indirect
evidence that ethylene is also involved in controlling TE development.
Recently, brassinosteroids have been shown to be necessary for the transition from
stage II to stage III of tracheary element differentiation (see below).
As
mentioned above, much of what is known about xylem differentiation at the
molecular level has been acquired using the inducible Zinnia elegans cell culture system. This system induces parenchymal
cells in culture to first de-differentiate and then to re-differentiate into
TEs (transdifferentiation). The
molecular markers identified in this system reflect its artificial nature in
that the de-differentiation phase is not usually present in normal TE
differentiation from protoxylem or cambial tissue. Hence the system has
more in common with wound-induced TE differentiation where pre-existing cells
undergo de-differentiation prior to
developing into TEs.
Stage
I: De-differentiation:
Using
the Zinnia mesophyll cell as a model,
this stage commences with the cells losing the ability to conduct
photosynthesis, the expression of wound-induced genes and the acquisition of
the ability to elongate and differentiate. Three groups of genes are
up-regulated during this stage, 1) wound-induced genes; 2) genes whose products
are associated with the protein synthetic apparatus and; 3) the remainder.
Stage II: Restriction of developmental
potential:
The
accumulation of TED2, 3, and 4 (Tracheary
element differentiation-related genes) gene products. This accumulation
occurs between 12 and 24 hours prior to the synthesis of the secondary cell
wall. These genes are also upregulated in vivo in procambial cells destined to
become TEs (TED3) or TEs or phloem elements (TED4 and TED2).
Inhibitors
of poly(ADP-ribose) polymerase, an enzyme necessary for DNA excision repair,
also inhibit the development of TEs. These same inhibitors also repressed the
expression of all TEDs.
There
is a marked increase in the transcript abundance of a number of genes whose
products are involved in the protein translational machinery which is
correlated with a dramatic increase in protein and RNA amounts present in these
differentiating cells. Additionally, tubulin gene expression increases,
providing the means of orchestrating secondary cell wall synthesis in the third
stage of development. Actin gene transcription increases as well, and large
cables of actin form along which cytoplasmic streaming occurs.
Stage III: TE specific development:
Brassinosteroids
are necessary for the transition from stage II to stage III of tracheary
differentiation. In this last stage of tracheary element differentiation the
secondary cell wall, necessary for the structural strength required to
withstand the high negative pressures exerted by transpiration without
implosion, is synthesized. The secondarily thickening of the cell wall occurs
by the synthesis of cellulose microfibrils perpendicular to the direction of
flow which, as in phloem, strengthen the element like hoops around a barrel.
Additional structural support is provided by cell wall proteins. An extensin
protein as well as an arabinogalactan protein are in high concentration in
mature tracheary elements. A characteristic alteration to the cell wall of the
tracheary elements at this stage is their heavy lignification. Programmed cell
death (see below) is tightly coupled temporally with secondary cell wall
thickening in this stage of xylogenesis. Finally, autolysis occurs culminating
in the generation of a cell corpse…a mature xylem element.
Apoptosis
(Programmed cell death (PCD)) vs necrosis:
All
cells die. How they do so varies. Some are slated for death internally,
genetically programmed to die a physiological death while others die due to
injury. Apoptosis or programmed cell death, is a process of
death from internal factors up-regulated in some cells during normal cellular
differentiation and development of multicellular organisms. This process is
also involved in tissue homeostasis, pathological conditions and aging. Cells
undergoing apoptosis are characterized by cell volume loss, plasma membrane
blebbing, nuclear condensation, and endonucleolytic degradation of DNA at
discrete intervals.
Not
all cells die through apoptosis. Dramatically traumatized cells such as those
suffering sever wounding or other overwhelming stress undergo necrosis, a non-physiological death
involving cell swelling, eventual lysis, and the leakage of the cell contents
into the intercellular space. Necrosis does not usually play a role in
differentiation and development and so will not be dealt with further.