PLS622
Plant Physiology I, Monday, October 2, 2006 Reproductive
development:
Transition
to flowering I:
The
environmental cues controlling the transition to flowering:
Plants
are extremely well attuned to their external environment. This is particularly
evident in the decision to commence reproductive growth following a period of
vegetative growth. Environmental cues such as temperature, daylength, indeed
any reliable measure of the passage of time or seasonality, can serve to permit
the synchronous flowering of a population of plants which is of crucial
importance for outcrossing species. What environmental factor or combination of
factors particular plants cue into to determine the switch to reproductive
growth and the organ change depending on the habitat the plant is growing in.
There are numerous examples of the same species of plant being say, biennial
with vernalization requirements in one environment and a long day annual with
no vernalization requirement in another. The signal that the environmental cue
elicits must often travel a considerable distance in the plant to reach the
vegetative meristem where it is to have its effect in converting the vegetative
to an inflorescence meristem.
In
order for a plant to respond to environmental stimula and change meristem
determination from vegetative to inflorescent 1) the leaves that are to detect
and/or transduce the signal must be competent to do so and, 2) the meristem
must be competent to change to an inflorescent meristem. The inability of some
long lived plant species to flower for the first few (or many) years is due to
a lack of competence to respond to environmental cues that usually bring about
flowering. This is termed juvenility and is a large thorn in the side of those
wishing to improve attributes of wood through genetic engineering for use in
the pulp and paper industry.
Vernalization: This term
literally translated means “springization”. It is the ability of a period of
low temperature to induce vegetative plants to flower. This can occur while the
plant is at the cold temperature (nondelayed
response) or after the plant has been removed to a higher, permissive
temperature (delayed response). It
can be necessary but not sufficient to induce flowering in plants requiring a
second (or more) inductive environmental signal prior to/after vernalization.
Some plants are responsive to vernalization as seeds, others as seedlings, or
mature plants. Additionally, as in cereals, the vernalization response can be
quantitative (facultative) where
treatment simply shortens the vegetative growth phase or as with Lancer wheat
where the response is qualitative (absolute)
since flowering does not occur without vernalization.
Biennial
plants often require vernalization to flower. The rosette of leaves of the first
year’s growth protecting the shoot apical meristem at their base during a
winter of cold temperatures which permits the production of a bolt and flowers
the following spring and summer, thus completing the life-cycle.
Either
high temperature or anoxia within 4 days of the completion of vernalization for
plants showing delayed response can cause devernalization where the plant loses
the effect of vernalization and fails to flower.
Photoperiod:
Photoperiodism was the first clear-cut demonstration of a biological clock.
Some plants will be induced to flower in response to day lengths in excess of a
certain minimum length (critical day)
so called long-day plants. Other plants respond to night lengths longer than
some maximum (critical night), so
called short-day plants. There are other plants that require long days followed
by short days as occurs in late summer and early fall these are the long-short-day plants. Their
counterparts are the short-long-day
plants that require short days followed by long days as occurs during the
early spring. Others flower only when day length is intermediate, remaining
vegetative if the day length is too long or too short. Still others flower
either under short or long days but not when day length is intermediate. The
rest of the plants are placed in the day-neutral category.
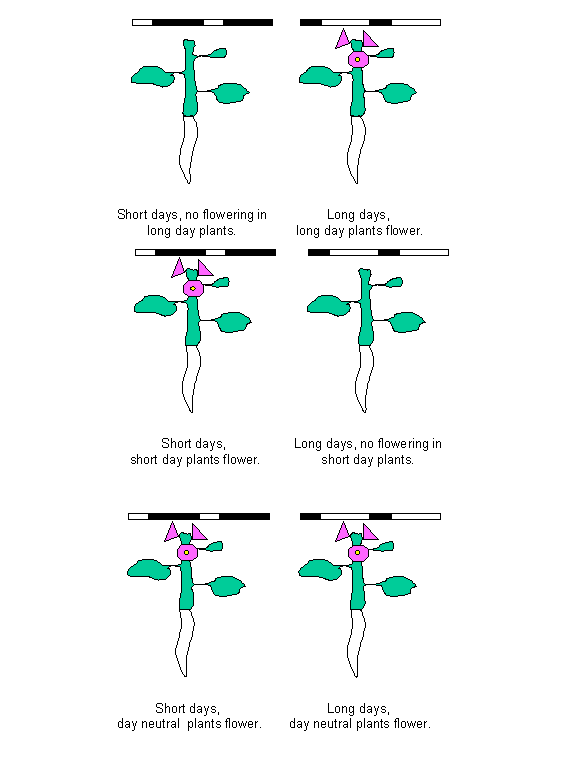
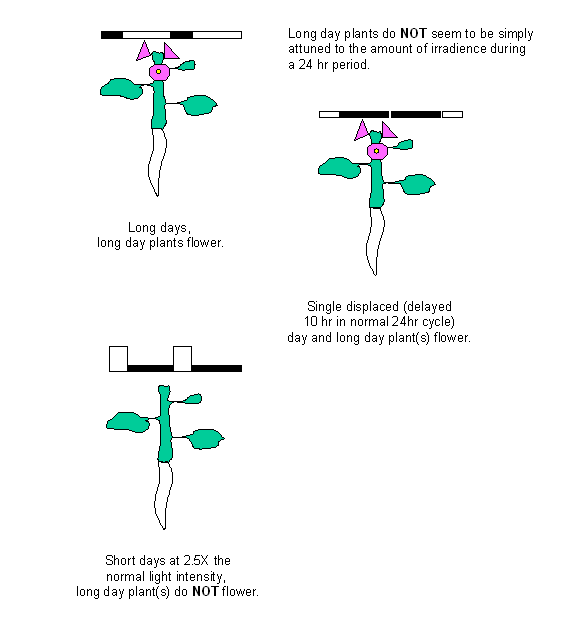 Light
quality:
Many of the day neutral plants can be induced to flower due to changes in the
intensity of the light they are grown under.
Light
quality:
Many of the day neutral plants can be induced to flower due to changes in the
intensity of the light they are grown under.
Whatever the
plant, it is generally agreed that the signal to switch from vegetative to
reproductive growth is a transmissable
signal.
There are three main hypotheses which
attempt to explain the nature of this signal.
1) The florigen/antiflorigen hypothesis suggests that the plant produces
either a yet to be discovered stimulatory or repressive compound in response to
conditions favorable or unfavorable to flowering, respectively.
2) The nutrient diversion hypothesis stipulates that a favorable flowering
environment is any environment that increases the amount of photoassimilate
available to the shoot apical meristem, converting it to an inflorescence
meristem.
3) Finally, the hypothesis of multifactorial control postulates that
combinations of assimilate and known phytohormones act synergistically to induce
flowering whenever environmental cues alter assimilate and hormonal ratios such
that they are favorable for the transition to flowering.
Plant torture: gaining insight through
surgical manipulation.
1) Plant-in-a-box: The fact that
the signal to begin reproductive growth is, in the case of photoperiod,
perceived by the leaves and transmitted to the shoot apical meristem has been
exhibited in many species by physiological experiments dating back to the last
century. The first such experiment involved inducing different parts of a plant
with one long day while the preponderance of the plant resided in a light-tight
container experiencing short days. When the exposed portion of the plant was a
mature leaf and this was induced with a long day, the result was the shoot
apical meristem took on the attributes of an inflorescence meristem.
2) Grafting: Stimulate one
plant with long days then excise a mature leaf at different times and graft the
leaf onto a plant that has been grown under non-inducing conditions. In Sinapis alba, the graft was often
sufficient to induce the non-induced plant to flower.
3) Defoliating: The second
such experiment is a “pulse chase” experiment where long day plants were
induced to flower by prolonged (greater than 8 hours) illumination (A.K.A. 'a
photoextended period') and subsequently defoliated at different hours after
induction. This latter experiment when performed on Sinapis alba exhibited that the slowest mobilized floral stimulant
exited the mature leaves between 12 and 16 hours after the commencement of the
long day.
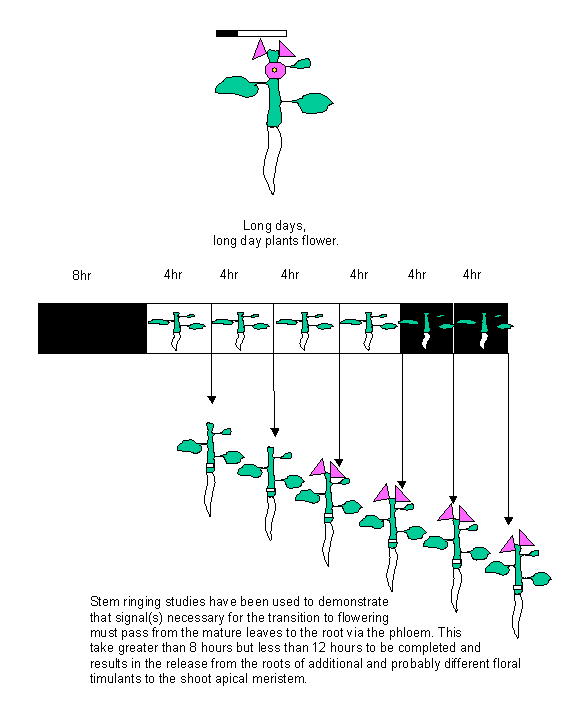
4) Stem ringing, stopping phloem
transport: See diagram below.
5) 100% relative humidity, stopping
xylem sap movement: Place un-induced plants in 100% relative humidity: A) 24
hours before a 24 hour constant light exposure; B) during a 24 hour constant
light exposure; C) 24 hours after a 24 hour constant light exposure. A and C
flowered while B did not. This means the signal is carried in the xylem at some
point.
The
possible nature of the floral stimulant:
Carbohydrates: By illuminating the long
day plant Sinapis alba for 8 hours at
light intensities 2.5 time greater than those usually used to induce flowering,
the overall accumulated irradience was equal to or greater than the amount
accumulated during long days. Yet the plant does not flower. This experiment
was used to demonstrate that long day plants are not simply responding to
accumulated illumination as the signal to flower. However, there were metabolic
changes that accompanied the exposure to high illumination that are identical
to those that take place upon the transition to flowering. The first of these
was an increase in carbohydrate amounts and an increase in acid invertase
activity in the apical meristem. This increase in sugar amounts, primarily
sucrose, precedes increases in energy requiring events such as mitosis and so
is not simply a reaction of the plant to provide more assimilate to a strong
sink. This has brought many to conclude that sucrose itself may be a signal
involved in the transition to flowering. The source of the carbohydrates
translocated to the apical meristem in response to higher than usual
illumination was stored reserves, primarily starch, in the mature leaves and
stem.
Cytokinins: Like the illumination of
long day plants with high intensity light of short duration (8 hours) a second
treatment that fails to induce flowering completely but does induce a sub-set
of events that are necessary for flowering is the application of cytokinin to
the apical meristem. This application stimulates the rate and synchronicity of
cell division along with some other cellular occurrences normally associated
with the transition to flowering seen upon photoperiodic induction. Induction
causes the concentration of cytokinins to increase in the mature leaves,
where both zeatin riboside (the major
component) and isopentenyladenine riboside (minor component) accumulate. The
supply of cytokinin has been traced to the roots of the plant and bark ringing
experiments have been used to demonstrate that the mature leaves signal to the
roots after induction and that preexisting cytokinin is released from the roots
and transported to the mature leaves through the xylem. This process takes
between 8 and 12 hours after the induction of flowering. The xylem has been
implicated in the transport of cytokinin from the roots to the mature leaves by
growing the plants for 24 hours in 100% relative humidity either before,
during, or after the induction of flowering. By growing the plants in 100%
relative humidity, among other things, the transpiration stream is much
reduced, delaying xylem transport. Only plants grown in 100% relative humidity
during the 24 hours which included the induction of flowering failed to flower
signifying that the message from the roots (cytokinin) is transported in the
xylem.
The
mature leaves, upon receiving the cytokinin from the roots are stimulated to
export isopentenyladenine (iP) primarily to the shoot apex. Levels of iP
increase in the shoot apex and in the mature leaves at about 16 hours after
induction of flowering. It is not known whether this increase in iP is due to
the leaves metabolising the imported cytokinin from the roots or if the
cytokinin from the roots is a stimulus used to promote the biosynthesis of iP
in the leaves.
Auxins:
Little
work has been done on the dynamics of auxin but it has been noted that there is
a pronounced decline in auxin concentration in the apical meristem at 16 hours
after induction of flowering, just when cytokinin concentrations are increasing
in this plant part. The auxin-to-cytokinin ratio is a potent cue for many
developmental processes in plants and the balance between them probably has
implications for events leading to the transition to flowering.
Polyamines:
Photoinduction
of flowering leads to a release of putrescine. Additional evidence implicating
polyamines in the induction of flowering are the observations that inhibitors
of polyamine biosynthesis, such as DL-a-difluoromethylornithine drastically reduce flowering
response.
Calcium:
Calcium
has been proposed to be a secondary messenger for flower induction in plants.
The levels of calcium in root exudates increase dramatically following
photoinduction. However, no increase in calcium amounts is found in mature
leaves or in leaf exudates. Despite this fact, calcium amounts are seen to rise
in the apical meristem approximately 40 hours after photoinduction. This
calcium is supplied to the apical meristem from the apoplast of surrounding
cells.
Synopsis:
The
work presented above has been discovered using the long day plant Sinapis alba as a model. Other plants
may utilize different signaling molecules. For example, the long day grass Lolium temulentum appears to use
gibberellins to signal flower induction whereas they are not used by Sinapis alba. It does appear as though
some short day plants (Xanthium
strumarium) utilize sucrose and cytokinins as signals for inducing
flowering as Sinapis alba does.