PLS 622 Plant Physiology I, Monday, September 11, 2006
Section II: Embryo and seed development:
Lecture
VIII: Fruit and Seed development:
IMPORTANT
TOPICS TO BE COVERED:
Fruit
development:
- We will learn about the four
different developmental phases fruit progress through from the time following
fertilization of the ovules in the ovary until the carpel(s) (in most cases)
ripen.
- We will learn about the the hormonal
messages (at least those we have discovered to date) that act as cues for each
of the four developmental phases.
- The different morphological classes
of "fruit" and what distinguishes them will be examined.
Seed
development:
- The different types of reserves
stored in developing seeds will be discussed.
- The four cardinal classifications of protein
based on solubility will be listed.
- We will examine the mode of and site
of accumulation of the 3 major stored reserves in developing seeds.
- The two fundamental divisions of
seeds, based on their ability to withstand desiccation and remain alive in the
dehydrated state, will be introduced.
Fruit development:
Upon
fertilization in many angiosperms, the carpel(s)
of the gynoecium usually develop into a fruit enclosing the fertilized zygotes
in an environment conducive to their development into mature seeds. The fruit
can also aid seed dispersal and, in some plants, need not develop from the
gynoecium. In so called pseudocarpic
fruit, receptacle bracts, the floral tube, or enlarged inflorescence axes
can contribute to the “fruit”.
Esau (1977)
classifies fruit into many different types based on whether the fruit is dry or
fleshy, dehiscent or indehiscent, true or false. This classification was
tabulated by Mauseth (1988) in 12 groups to which I have added a 13th
(number 8 in the table) and excerpted below as Table 1.
When
the ovary of the gynoecium develops into a fruit the ovary wall is termed a pericarp. The ovary of tomato is such a
fruit with two, three or four carpels (depending on the species and variety of
tomato) fusing to form it. The number of cells recruited from the L3 layer
(Fig. 1) in the shoot apical meristem into the inflorescence meristem
determines the size of the floral meristem and the number of carpels it is to
produce. It is the L3 layer that gives rise to the
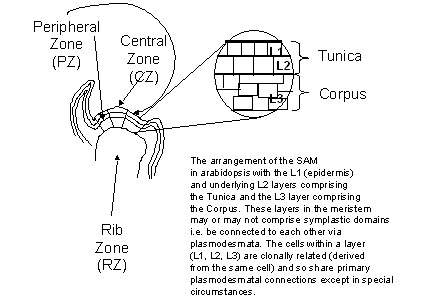
Figure
1:
The three zones of the shoot apical meristem (SAM).
preponderance of the pericarp tissue
and this may be the reason why the L3 layer dictates the
final carpel number in tomato. The
argument runs that, genetically, the cells of the L3 layer will control how great
a sink the developing fruit is and therefore, how much assimilate will be
potentially available to the fruit and enclosed seeds.
The
fruit of tomato consists of two to four carpels that fuse and develop into a
pericarp. Developmentally, the carpel can be compared to a leaf that has curled
around upon itself and houses the ovules. The cells of the carpel of tomato
most resemble those of the palisade layer of the leaf and maintain their
chloroplasts; functional chlorophyll, and photosynthetic capacity well into
fruit development. Although it is not substantial, the fruit photosynthesis
does contribute to photoassimilate required for fruit development. The carpel
expresses many of the genes that are typically associated with leaves, albeit,
only a sub-set of so-called “leaf specific” genes and often at stages of
development divergent from that of the leaf.

There
are at least four (arguably 5) discernable periods or phases of fruit growth,
excluding processes occurring prior to pollination and fertilization. Those
over which there can be no debate include: 1)
fruit set; 2) cell division, 3) cell enlargement (both isotropic and
anisotropic cell growth) and; 4)
maturation (ripening). The controversial 5th phase is
senescence.
Phase
I: Fruit set: Although little is known about the control of ovary
development into fruit, it is known to usually be dependent on successful
pollination or pollination and fertilization of ovules within the ovary. Some
signal is released between the time of pollination to the time of
fertilization, perhaps throughout, that avoids ovary abortion and sets the
stage for the second phase of fruit development, cell division. This positive
signal may be gibberellic acid (GA) a.k.a. gibberellin, for this hormone is released by the pollen, the
pollen tube and the fertilized ovule. Exogenously supplied GA can promote parthenocarpic fruit production in
tomato. A parthenocarpic fruit is one that develops to maturity; a) without
pollination (banana); b) without fertilization (some orchids); or c) with
pollination and fertilization but subsequent abortion of the ovules (grapes).
The lack of maintained, fertilized ovules results in a fruit that, at maturity
is devoid of seeds. However, the GAs produced by parthenocarpic fruit are not
the same set as those produced by normally fertilized fruit. *Additionally,
applied GA stimulates auxin (a
second plant hormone) production by the ovary, and auxin too could participate
in signaling the ovary to avoid abortion. It is possible that parthenocarpy is
a direct result of improper synthesis of auxin both temporally and spatially
within the ovary. This suggests that the synthesis of GA and auxin during ovary development must be highly regulated in
order to coordinate both fruit set
and the commencement of cell division…the next phase of fruit development.
When
there is little GA produced in the ovary due to a lack of
pollination/fertilization, the shoot apex appears to inhibit expansion of the
ovary. This may be due to basipetal transport of auxin (IAA) from the apex of
the shoot to the ovary and this may trigger
Phase II: Cell Division: In normal fruit development it is the developing
seed that controls the rate of cell division in the surrounding tissue. Phase
II lasts between 7 to 10 days during which cell division occurs throughout the
fruit. Mitotic activity is high initially in the whole pericarp, but more so in
the outer relative to the inner pericarp. Additionally, cell division is
initially higher in the integumentary layers of the developing seeds than in
the embryo. The developing vascular trace in the placenta also exhibits high
cell division activity early during phase II. At the middle of phase II,
mitotic activity is restricted to the outer pericarp, developing seeds,
vascular tissue, and placenta proximal to the developing seeds. At the end of
Phase II, cell division remains the same in the pericarp as it was in the
middle portion of Phase II, Cell division in the placenta is also restricted to
the outer layer of cells from which the locular tissue arises. The vascular
tissue and the developing embryo also exhibit high mitotic activity.
There is a general observation that
the number of fertilized ovules within a fruit controls the initial rate of
cell division in the ovary. This is exemplified by the observation that if
seeds are produced on one side of a fruit but not on the other, the fruit
becomes lop-sided with the larger lobe containing the developing seeds. An
additional correlation perhaps explaining the first is that the amounts of cytokinin (the third plant hormone known to be involved in fruit development [
Phase
III: Cell expansion: This stage increases the fruit far beyond its size during
the previous two stages through the controlled expansion of the cells formed in
the previous stage. The primary hormonal stimulant driving fruit cell expansion
is auxin and the hormone peaks twice during Phase III. The first
peak indicates the onset of phase III and auxin is most prominent in the
developing seeds. However, in parthenocarpic fruit, exogenously supplied auxin
is not sufficient to replace developing seeds as a stimulant of cell expansion.
This has lead to the belief that it is the seeds’ ability to act as intense
sinks that permit access to photoassimilate that is also used for fruit
development. Alternatively, it is possible that high seed auxin concentrations
promote the production of another stimulatory molecule within the seed that
diffuses or is transported into the surrounding tissue and stimulates cell
expansion. Because seeds are not present in parthenocarpic fruit, applied auxin
would not stimulate the production of the second compound and would therefore
be insufficient to stimulate fruit expansion. This scenario is further
supported by the observation that the tomato mutant diageotripica (dgt) (exhibiting
attributes of auxin-deficiencies in its growth) produces normal fruit.
The
second peak of auxin coincides with
the end of the fruit expansion phase
occurring at the period of maximum embryo expansive growth. Again, auxin levels
are high in the seed but low in the fruit and is absent in parthenocarpic
fruit. This peak is associated with the cell expansion occurring in the embryo
since the fruit cells are at their final size.
A
second plant hormone that may play a role in fruit development is gibberellic acid (GA). As with auxin there are two peaks of GA occurring during fruit
development one during Phase II the second during Phase III. In Phase II, GA
peaks at the onset of cell division. In Phase III GA peaks, possibly due to
auxin stimulation of GA production, during cell expansion, at maximal fruit
growth. However, parthenocarpic fruit exhibit an exaggerated first GA peak in
the absence of seeds that are the main sites of auxin concentration in the
fruit (see above).
Both
the rate of fruit development and final fruit size are controlled by cell
number and the sink strength of the cells comprising the fruit. The affect of
cell number may be a function of a greater cumulative sink produced by many
versus few cells. This is apparent when the timing of fruit set on a truss is
altered. Normally fruit set occurs basipetally and the first fruit to set are
those proximal to the main axis of the plant and these fruit contain more cells
than do fruit that set more distally along the truss. If fruit are prevented
from setting proximally until after more distal fruit have set, the more distal
fruit have a greater rate of development despite having fewer cells. In
addition to metabolic control of fruit development, there are indications that phytosterols are also responsible for
early events in fruit development. Injection of an inhibitor of hydroxy-methyl CoA reductase (HMGR)
results in arrested fruit development. However, whether phytosterols themselves
are responsible for the observed effects or whether it is a lack of the
cytokinin and gibberellic acid produced from the mevalonic acid common to both
pathways is unknown.
One
noticeable morphological trait becoming obvious during fruit expansion is an
increase in the width of the peduncle (stem of an inflorescence of flowers) and
the pedicle (attachment of the flower [and now the fruit] to the plant). This
is accompanied by an increase in the amount of the vasculature in these organs
but this is not easily observed. Can you
think of why such an increase may be necessary for the expanding fruit?
Phase
IV: Maturation (Ripening): There are two different types of fruit based on their
physiological behavior during the maturation (ripening) phase. Some fruit show
a considerable burst of respiratory activity at the onset of ripening. These
fruit are said to be climacteric,
evolving considerable CO2 and the plant hormone ethylene (the fourth plant hormone known to be involved in fruit
development) during this phase. Climacteric fruit include apple, banana and
tomato.
Other
fruit do not undergo a climacteric burst of respiration (CO2
production) and ethylene evolution. The fruit of strawberry, citrus and
pineapple belong to this class. Ethylene has long been known to be a potent
stimulant of maturation in climacteric fruit. Many genes whose products are
implicated directly in fruit ripening are positively up-regulated by ethylene.
These ripening associated processes include: 1) partial cell-wall disassembly resulting in decreased fruit
firmness; 2) a change in the composition
of fruit volatile substances resulting in altered fruit aroma and flavor; 3)
a change in pigmentation resulting
in altered fruit color and; 4) an
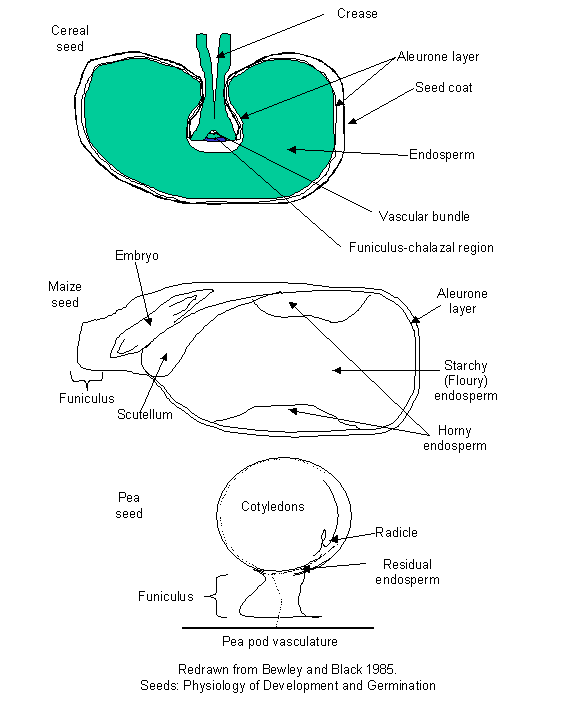
Figure
2:
Some anatomical features of mono- and di-cot seeds.
increase
in
fruit respiratory activity. Ethylene
biosynthesis itself is controlled by ethylene in climacteric fruit in a
positive feedback loop known as “auto-catalytic”
ethylene production. This biosynthetic pathway is known as System II to
distinguish it from the non-auto-catalytic biosynthetic pathway operating in
vegetative tissue (System I). In tomato the dominant mutation Never-ripe (Nr) is defective in an ethylene receptor and produces fruit that
stay green and firm. One of the ethylene-inducible enzymes responsible for
partial cell wall disassembly during ripening-induced fruit softening
(polygalacturaonse) has been antisensed to produce commercial varieties of
tomatos that maintain a long shelf life (Flavor saver). In arabidopsis there
are at least 5 receptors for ethylene, all of which are negative regulators of
the ethylene response. In the absence of ethylene, these receptors send signals
that actively repress responses to ethylene. When ethylene is present, the
receptors cease to send their message and the result is a response to ethylene.
See Anthony Bleecker's excellent review
on this subject in Trends in Plant Science, 4: (7): 269-274.
The
physiological significance of climacteric fruit ripening is not understood. However,
it has provided a convenient means of controlling fruit ripening by decreasing
the amount of and sensitivity to ethylene. Thus, a highly perishable crop such
as bananas can be picked prior to maturity, and shipped under conditions that
minimize ethylene production/sensitivity, and then artificially ripened at the
point of sale by exposing fruit to ethylene.
Seed
development:
Our knowledge
of the commencement of seed development (embryogeny and storage tissue
formation) has been discussed by Dr. Perry over the course of the last few
lectures. I will continue this discussion on the latter stages of development
focussing on the synthesis and deposition of reserves to fill the cells of the
storage tissues and the phases of seed maturation.
Basic
anatomy:
Deposition of reserves in the storage tissue of seeds is the hallmark of every
seed known, providing material for energy and early growth. The assimilate for
storage reserve synthesis is translocated from the mother plant into the sink
cells of the seed. The nature of the reserves laid down as well as the tissue
in which they are laid down delimits major seed characteristics as well as
morphologically distinguishing features used to classify plants. One of the
most fundamental differences is related to whether the embryo develops one or
two cotyledons (or many cotyledons as in the Pinaceaea). This trait has
permitted a sharp differentiation between two classes of Angiosperm, the monocotyledones (one cotyledon) and dicotyledones (two cotyledons). The
single cotyledon of the monocots (the scutellum
Fig. 2, 4A) is usually of a secretory and absorptive nature, never exiting the
seed proper, even after germination is complete. It abscises from the seedling
and is shed along with the exhausted endosperm
and testa upon the completion of
seedling establishment. It rarely contains substantial amounts of stored
reserves since it is usually associated with endospermic seeds (seeds in which the major storage organ is an
endosperm). Dicotyledonous embryos can be found in endospermic and
non-endospermic seeds. Many dicots remain endospermic but some (e.g. Pea)
absorb the endosperm during development, redistributing the reserves within the
cotyledons (Fig. 2). Endospermic seeds may retain live endosperm cells while in
other endospermic seeds the endosperm is dead at maturity. In the latter
instance, a thin layer of live, non-storage, secretory cells often surrounds
the endosperm along the periphery of the seed, inside the testa. This is known
as the aleurone layer and aids in
digesting the endosperm to provide the embryo with the nutrients stored in the
dead endosperm (Fig. 2).
Reserve
deposition in storage tissue: There are usually only two of three
possible major storage reserves
deposited in seeds as well as several minor reserves. The major storage
reserves are: 1) Protein; 2) Lipid and; 3) Complex Carbohydrates (Starch, Fructans, diverse
Hemicelluloses). Storage protein is found ubiquitously in seeds as one form of
major storage reserve comprised of nitrogen and carbon along with either
polysaccharides (usually starch) or lipid, never both in major quantities, as
the second major reserve. So, some seeds house protein and primarily
polysaccharides, while others house protein and primarily lipids. These major
reserves are extensively hydrolysed only after the completion of germination
and the seed depends initially on a minor reserve of soluble carbohydrate
(sucrose, and often the raffinose family oligosaccharides (RFO)) for initial
metabolism and structural molecules prior to radicle protrusion. Additionally,
essential macro- and micro-nutrients are sequestered in the seed, usually in a
complex with hexaphosphorylated myo-inositol
named phytin or phytic acid located as an aggregate embedded in storage protein in
the protein storage vacuole/protein body. How are these reserves deposited in
the seed?
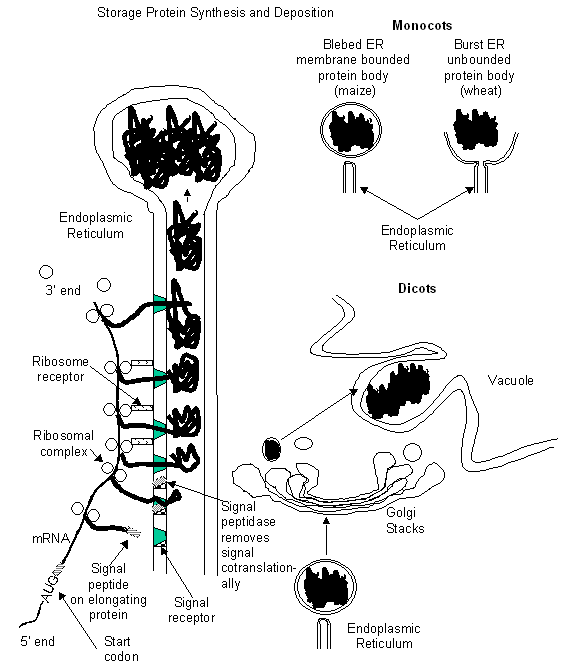
Figure
3:
Synthesis and deposition of storage proteins in mono- and di-cots.
Storage
protein deposition: During seed development, massive (relatively speaking)
amounts of specialized proteins are synthesized for use as a source of
nitrogen, amino acids, and energy during seed germination and subsequent
seedling establishment. There are many families of storage proteins that can be synthesized and stored in the same
seed. They are often oligomers of
several different polypeptides and frequently exhibit different physical
characteristics most notably regarding solubility. Hence, seeds can have
storage proteins that freely dissolve in water
(albumins), those that dissolve in
buffers of high ionic strength but
not
B. A.
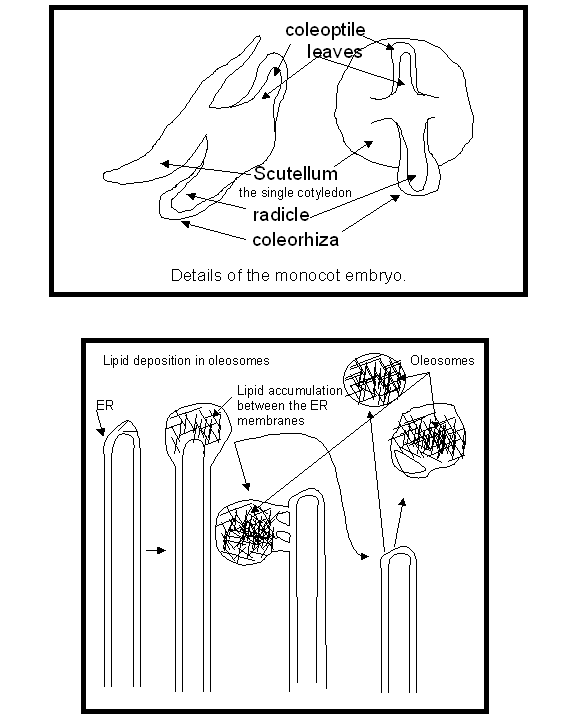
Figure
4:
A) Details of the embryo of many monocots (maize is featured). B) A schematic
of the deposition of lipid in oleosomes.
water (globulins), those dissolving in aqueous (70-90% v/v) alcohol (prolamins)
and those requiring dilute buffers of either high or low pH to solubilize (glutelins).
The storage
proteins are all synthesized on the rough endoplasmic reticulum (ER) and are
deposited in the lumen of the rough ER. The storage proteins are placed in the
lumen by virtue of an amino-terminal signal
peptide that directs the nascent protein through the ER membrane while the
protein is still being translated from the storage protein mRNA in the
cytoplasm. The signal peptide binds with a special docking protein in the ER
membrane and is cleaved off the nascent protein co-translationally. Upon deposition of the full-length protein into
the ER lumen, the ribosomal complex that has orchestrated the translation
disassembles from the mRNA and disassociates from the ER (Fig. 3). The storage
protein aggregates within the ER lumen and is moved to a peripheral terminus
that then has various fates depending on the class of plant (Monocotyledones vs
Dicotyledones).
Monocotyledonous
storage protein deposition: In monocots, the protein body may be simply an aggregate of the storage proteins
that have stretched the ER until it ruptured, releasing the aggregate into the
cytoplasm unbounded by any membrane (e.g. wheat, Fig. 3). Alternatively, the
storage proteins can accumulate until the ER terminus eventually blebs off and
forms a lipid bilayer surrounding the protein in the protein body (a unit
membrane) (Fig. 3).
Dicotyledonous
storage protein deposition: The storage protein aggregated in a
terminus of the ER in dicots accumulates until the ER terminus blebs off from
the ER. However, the ER vesicle containing the storage protein is then
transported to the Golgi apparatus
where it fuses, releasing the storage protein into the Golgi. Considerable
processing of the storage protein can occur in the ER and the Golgi usually in
the form of glycosylation, partial or complete assembly of oligomeric
polypeptides, the formation of disulfide bonds, and/or the cleavage of
cotranslated subunits. The protein is passed from the proximal stacks to the
distal in the cisternae until it is packaged in a Golgi vesicle and transported
to the vacuole. The vacuole of the dicots during seed development can either
remain as a single, central vacuole or, more commonly, become highly
convoluted, pinching off small vacuoles that are filled with storage protein
and phytin. Regardless, they are referred to as protein storage vacuoles to reflect their origin and not as protein
bodies (Fig. 3).
Storage
lipid formation and deposition: Many, but not all, seeds deposit lipid
for use as an energy and carbon source during germination and seedling
establishment. Lipid is synthesized in the seed from assimilate sucrose
delivered to the seed from the mother plant. It is converted via the pentose
phosphate pathway-, glycolytic-, and the triglyceride biosynthetic
pathway-enzymes located in the cytosol and proplastids
into three primary free fatty acid precursors of the large diversity of lipids
stored in seeds (Palmitate (16:0), Stearate (18:0), and Oleate (18:1)). From
the proplastids, these precursors are transferred to the endoplasmic reticulum
where they undergo a myriad of alterations (elongation, addition of double
bonds, hydroxylation, addition to glycerol to form triglycerides, and temporary phosphorylation) before being bulked
and packaged in membrane vesicles formed from the ER, the oleosomes (lipid bodies). There is a variety of oleosome forms, all
arising from deposition of lipid in the ER but in different manners. The
oleosome can remain attached to the ER though thin membrane channels, or bud
off forming an oleosome bounded by a single layer of ER membrane (a half unit
membrane) that either does or does not have an extension of ER membrane
attached to it (Fig. 4).
Storage
polysaccharide formation and deposition: Polysaccharides constitute a
third form of major storage compound. Usually, seeds storing carbohydrate do
not also store major amounts of lipid. By far the most common form of
polysaccharide used by seeds as a storage reserve is starch. Starch is formed in proplastids by the daily deposition of amylose (unbranched chains of a-1-4 linked
glucose) or amylopectin (chains of a-1-4 linked glucose with branches of a-1-4 linked
glucose chains attached to the main chain through a-1-6 links).
The proplastid, termed the amyloplast,
is filled with a single grain or numerous grains of starch.
In
other seeds, the major polysaccharide deposited is fructan, galactomannan,
or xyloglucan. These reserves,
except for fructan, are deposited outside the cell as a secondarily thickened
cell wall. In some seeds such as tomato, the secondarily thickened cell wall of
the endosperm does not affect the cells themselves while in other species, e.g.
fenugreek, the secondary thickened cell wall eventually occludes the cytoplasm
and kills the cell.
Phytin
synthesis and deposition: Little is known about phytin
synthesis or deposition. Phytin is a water-insoluble accumulation of a mixed
salt of magnesium, calcium, and potassium ionically bound to myo-inositol
hexaphosphoric acid (phytic acid). It can also contain iron, manganese, and
copper, and sodium salts. Current evidence suggests that phytin is synthesized
in the ER and, upon sufficient accumulation, an ER vesicle buds from the ER and
fuses with the protein body or protein storage vacuole and deposits the phytin
in the globoid (the aggregate of
phytin embedded in the storage protein).
Soluble
carbohydrate (oligosaccharide) synthesis and deposition: The
non-reducing sugar sucrose is usually found in considerable quantities in
mature seeds. The non-reducing nature
of sucrose along with its versatility, being quickly converted into energy
and/or carbon skeletons for use in germination and/or seedling establishment,
enables those seeds that do desiccate to maintain a quick energy source and do
so without incurring non-enzymatic reactions of cellular contents with the
reducing end of a sugar. These so-called Amadori and Maillard reactions
(non-enzymatic reactions between proteins and carbohydrates) result in
deleterious denaturing of essential cellular components that would severely
damage if not destroy the seed should they occur (they are responsible for the
browning of food during cooking). Sucrose, when present in large quantities,
during the removal of water tends to crystallize out of solution which
constitutes a quandary for seeds undergoing maturation drying. How to keep the
sucrose from crystallizing as it becomes more concentrated due to water loss?
Typically, the inclusion of some of the raffinose series oligosaccharides
(sucrose with one or more galactose moieties added to them) provides sufficient
disruption of the sucrose crystalline structure to prevent crystallization and
instead leads to an amorphous, highly viscose solution or glass. It is thought
that those seeds that have the ability of losing most of their water and yet
maintain life (anhydrobiosis) do so
by substituting sucrose and raffinose for water in providing a shell of
hydrogen bonding substances around membranes and proteins. This serves to
preserve most of the intracellular structural organization until such time as
water is reintroduced by imbibition.
Maturation
desiccation: A fundamental difference between the seeds of two classes
of plants is exhibited at this stage of seed maturation. Those species whose
seeds naturally undergo drastic water loss upon maturing are referred to as orthodox seeds and represent the vast
majority of the angiosperms. Those species whose seeds do not undergo
desiccation upon reaching maturity are designated as recalcitrant. Recalcitrant seeds that are dried do
not survive. There have been further classifications based also on the ability
of the seeds to survive cold temperatures but these are less clear than the
orthodox and recalcitrant designations.
For
orthodox seeds maturation desiccation
is necessary for the seed to subsequently complete germination normally. Seeds
that do not undergo maturation drying typically stay in the maturation phase of
reserve synthesis and deposition and fail to complete germination. Hence,
desiccation is considered as a physiological and genetic switch from maturation
to germination phases of a seed's development. Recalcitrant seeds do not
undergo sever maturation desiccation and yet are capable of making the switch
from the maturation to the germination programs. It has been suggested that recalcitrant seeds
do decrease in water content slightly upon maturation and that this is
sufficient to trigger the switch to the germinative mode.
It
is important to realize that most forms of major storage reserve exist in the
seed as large polymers of insoluble material (ergastic substances). This allows
the accumulation of sufficient stored reserves to provide the embryo with food
and energy to establish and become autotrophic without incurring the huge
negative osmotic potential that would be associated with such huge amounts of
reserves if they were osmotically active (i.e. soluble and unpolymerized). The
hydrolysis of these reserves to make them available for utilization will
therefore, require the utmost control so that the osmotic potential of the cell
does not become too great, resulting in excessive turgor pressure leading to
cell rupture and death.