Agr. Med. Vol. 130, 103-112 (2000)
|
ROOT DEVELOPMENT OF FLOAT SYSTEM TOBACCO PLANTS AFTER TRANSPLANTING |
L.V. Caruso, R.C. Pearce, L.P. Bush
Kentucky Exp. Stn, Kentucky, USA; Agronomy Dept., University of Kentucky, Lexington, Kentucky, USA,
paper no. 99-06-158
SUMMARY. In the past few years, there has been a strong shift in tobacco (Nicotiana tabacum L.) transplant production from traditional outdoor plant-beds to polystyrene cell trays floated in nutrient solution. Media and water roots produced by tobacco transplants were studied and their contribution to seedling establishment at 2 and 4 weeks after transplanting were investigated. Four weeks after transplanting seedlings to greenhouse pots, water roots did not contribute significantly to tobacco plant establishment. A strategy to eliminate water roots and to improve transplant development in the float system and performance after establishment in the field was developed. To stimulate production of a greater mass of media roots, the bottom of some trays were covered with a water permeable fabric and treated with a chemical root-pruning paint containing 100 g
Cu(OH)2 L-1 prior to filling with soilless medium. Greenhouse and field trials were conducted. Seedling development in trays treated with Cu
(OH)2 resulted in plants with a fine and longer root system than control plants. Seedlings from trays treated with
Cu(OH)2 had approximately 60% greater root length 4 weeks after transplanting in greenhouse pots. In field studies, transplants grown in
Cu(OH)2 treated trays had greater root length per volume of soil in the 0-30 cm depth than control plants. The greater early root growth of seedlings after transplanting may improve water and nutrient uptake that may aid transplant establishment in the field.
Key words: copper, chemical root pruning, SpinOut, transplanting establishment
|
INTRODUCTION |
The float system has become the major production system for tobacco (Nicotiana tabacum L.) transplants in the United States. An ideal transplant is disease-free, hardy enough to survive transplant shock, and available to be transplanted on time (SMITH, 1998). In this production system, tobacco transplants produce two distinct kinds of roots. The
media roots are roots that grow in the substrate within the float tray cell. They have an appearance similar to roots produced by plants grown in the traditional plant-bed system. The second kind is often called
water roots. These are roots that grow out of the bottom of the tray into the nutrient solution and differ in appearance from the media roots. Water roots tend to be very fragile and are easily broken off or injured as the plants are removed from the tray during
transplanting. Field establishment of the young tobacco plant is dependent upon growth or new formation of the media and water roots in order for the plant to acquire necessary nutrients and water supplies.
The most critical period in the development of tobacco plants occurs immediately after transplanting into the field. The young plants are removed from the protective environment of the float bed system and are subjected to radically different and sometimes adverse field conditions, resulting in stress on the juvenile plants. Bare-root transplants from conventional plant-beds have an extreme size imbalance between the root system and the shoot that often results in an extended period of stress and slow growth, called transplant shock. This shock at transplanting is caused by the loss of most of the root system in the pulling process (HOYERT, 1979). Climate adversity is also responsible
for transplant shock severity. This is particularly true in Kentucky were warm moist spring conditions abruptly change to hot dry summer conditions. Water requirement of the crop is high during seedling establishment in the field and a large functional root system helps provide water and nutrients for this critical phase.
Little care is taken with the water root portion of tobacco seedlings during the transplanting of young plants to the field. In most instances, when tobacco farmers handle the float trays, they ignore the bottom part of the seedling roots which results in complete loss of the water roots. Thus, pruning the water roots could affect the early days of transplant establishment in the field.
Tips of water roots are less metabolically active than media root tips, although water roots can be very long and account for 30% of the total root system length prior to transplanting time (CARUSO, 1999). The main root axis has been reported to regulate the development of branches (WIGHTMAN and THIMANN, 1980). This is particularly true for water roots because despite their length, they do not produce a great amount of branches along the main root axis (CARUSO, 1999). In contrast, lateral roots (branches) are more numerous or grow longer and faster when the main axis is prevented from elongating or is removed (RIEDACKER
et al, 1982). Based on the metabolic activity and growth pattern, media roots are apparently more important than water roots for seedling and transplant development. Therefore, we suggested that eliminating the water roots would improve media root growth and subsequent tobacco transplant establishment in the field.
Cupric hydroxide and cupric carbonate are treatments that effectively control root growth in a variety of plants (ARNOLD
et al, 1993). These chemical compounds have also been applied to the inside of seedling containers to increase root branching (fibrosity of roots) during seedling production (ARNOLD
et al, 1989, 1991). Tomato (Lycopersicon esculentum Mill.) seedling dry
weight was reduced by Cu(OH)2 - treatment of the container flats (LATIMER et al, 1993). However, after transplanting total root dry weight and shoot dry weight gain were greater for seedlings from
Cu(OH)2 - treatments compared to seedlings from non-treated containers. Nursery plants grown in copper-treated containers were observed to establish in the landscape more quickly than traditional-grown container plants (ARNOLD and STRUVE, 1989). More rapid establishment and resumption of growth of transplants would also be of benefit in tobacco production.
Most significant to efficient establishment of transplants is to know how the water roots and the media roots function in the first few days of transplant establishment. The contribution of each of these kinds of roots for nutrient and water uptake in the field is not known. The objectives of this study were: 1) assessment of tobacco transplant growth with or without water roots, two and four weeks after transplant; and 2) determination of the effect of chemical root pruning using a copper hydroxide-based product on subsequent transplant root development and plant establishment after transplanting.
|
MATERIALS AND METHODS |
Transplant Production. Burley tobacco seedlings (var. NC-129) were grown in the greenhouse of the College of Agriculture at the University of Kentucky, Lexington, KY. Pelleted seeds were sown in trays and floated on beds of nutrient solution. The growing medium was conventional non-fertilized and peat-vermiculite based soilless medium. Polystyrene trays (67.5 by 34.5 by 7.5 cm) were used, each containing 200 cells. The cells were open-ended with a
volume of 27 cm3 in an inverted pyramid shape.
Trays were manually filled with premoistened medium. Pelleted seeds were seeded
with a vacuum seeder into a depression or dibble (round shape of 12.5 mm depth).
Care was taken during seeding to ensure that one seed was placed in each cell and the seed was at the bottom of the dibble. Temperature at the tray level was maintained at 20 to
24o C. The water tank was fertilized with 20-10-20 (N-P2O5-K2O) water soluble fertilizer at a rate of
100mg N L-1.
Experiment 1: Assessment of transplant growth with and without water
roots.--- Polystyrene tray cells were cut and seedlings were removed carefully to preserve both the media and the water roots. Seedlings were planted in round plastic pots (20 cm top diameter; 14 cm bottom diameter, 22 cm height) filled with 3 kg of a 2:1 soil:sand sterile mixture. A physical barrier was placed in the middle of each pot to separate and identify new root production from the water roots and from the media roots. Thus, each pot was divided into upper and lower parts by a water-permeable barrier made of black landscape fabric called Weed-X. Seedlings were planted over the barrier either with intact water roots attached or with water roots totally removed. Intact water roots were threaded through a small hole in the middle of the barrier. Plants were randomly placed on benches in a greenhouse and watered regularly (2 L
day-1 pot-1) until the end of the experiment. Evaluations of root development were made two and four weeks after transplanting into the pots. Pots were cut open and the soil/sand mixture washed from the roots. Roots were separated into those growing above the physical barrier called upper part, and those found below the physical barrier, called lower part. Root systems from both parts of the plastic pots were suspended in water and stored at
5oC for root length and diameter measurement.
Experiment 2: Effect of chemical and physical root pruning of tobacco transplants, greenhouse trial. In a greenhouse trial, three tray treatments were used: 1) A commercial root inhibiting copper hydroxide product was applied to the inside walls of the tray cells and a barrier fabric was affixed to the bottom of the tray (chemical and physical barriers
combined - Cu+Cloth); 2) A water permeable root barrier fabric was affixed to the bottom of the tray (physical barrier only -Cloth); and 3) Untreated control (regular float trays). Each tray was sub-divided in three parts in a such way that all treatments could be applied in the same float tray. Before seeding, two-thirds of the external bottom part of the tray was covered with the water permeable landscape fabric described in Experiment 1. An acid-free multi-purpose spray glue was spread at the bottom of the tray and allowed to dry for few minutes before affixing the fabric. The interior and the bottom surface of one-third of the cells were painted by hand with a gray latex paint called Spin Out (Griffin Corp., Valdosta, GA), containing
100g Cu(OH)2 L-1 (7% w/w), and allowed to dry. There was no reduction in the drainage hole size in the bottom of the tray. All the trays were filled with soilless medium substrate and seeded following a standard procedure as described above.
Upon reaching transplant size, seedlings were carefully removed from the treated trays and transferred to plastic pots (20 cm top diameter; 14 cm bottom diameter, 22 cm height) containing a 2:1 soil:sand substrate and allowed to grow for four weeks. Water roots were removed from the untreated control plants prior to transplanting. Pots containing the transplanted seedlings were randomly distributed on greenhouse benches and watered regularly (2
L-1 day-1 pot-1) until the end of the experiment. Four weeks after transplanting into the pots, roots were evaluated following removal of the soil:sand substrate.
Experiment 3: Effect of chemical and physical root pruning of tobacco transplants, field trial. For this experiment the three tray treatments of Cu+Cloth, Cloth and Control were carried out as previously described. A fourth treatment included the Cu product painted on the inner surface of the cells, but the bottom of the tray was not blocked. In this
trial, tobacco seedlings were taken to the field. Seedlings grew to transplant size approximately seven weeks after seeding. A field trial started on the 5th of May at the University of Kentucky Experimental Farm (Lexington-KY) on a Maury silt loam soil (fine, mixed, mesic typic paleudolf). Root analysis was conducted by randomly selecting four plants per treatment. Each root sample consisted of four sub-samples taken 10 cm from each side of the plant. Soil samples were taken to a depth of 30 cm and separated into 0-15 cm and 15-30 cm cores. Samples were placed in plastic bags and kept in a cold room until roots could be washed and separated from soil particles for further measurements. In addition, the upper parts of the plants were cut for dry weight determination.
Leaching and residual copper concentration in the float water was also checked. Copper levels were determined by atomic absorption spectrophotometry at several times during seedling development.
In all experiments, shoot and root dry weights were determined for each sampled plant after being dried in an air-forced oven at 65°C. Evaluation of root systems was achieved with the aid of Mac/WinRHIZO (Regent, Inc.), a computer imaging and analysis program (CARUSO, 1999). All data were subjected to analysis of variance (ANOVA) procedures for a completely randomized design (SAS Institute, 1996). Least significant differences (LSD) were utilized to separate treatment means when ANOVA indicated significant (P< 0.05) treatment differences.
|
RESULTS AND DISCUSSION |
Experiment 1. The landscape fabric barrier placed in the middle of the pots to separate media roots from water roots was a useful tool. The total root system of plants was divided in two regions: upper (above the barrier) and lower (below the barrier) parts. However, when the pots were cut open four weeks after transplanting, some media roots were found below the barrier in
the pots. Media roots after touching the fabric,
grew laterally toward the outer edge of the pot around the edge of the barrier
and downward into the bottom area where the original water roots were located.
These roots were considered to have originated from the media root despite being
found below the barrier, but were included in the lower root total.
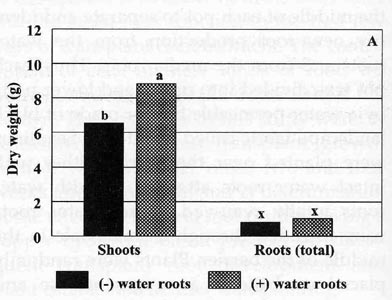
The plants with water roots had a significantly higher
shoot dry weight (8.8 g plant-1) than plants without water roots
attached (7.5 g plant-1) two weeks after transplanting (Fig. 1A). There was no
significant difference in
|
|
|
| Fig. 1
Dry weight accumulation of tobacco plants 2 weeks (A) and 4 weeks (B)
after transplanting to greenhouse pots. Roots (total) = upper+lower
pot parts, (-) water roots = plants without water roots attached; (+)
water roots = plants with water roots attached; bars represent mean
values; means followed by the same letter are not significantly different
from each other using t test (LSD) at 0.05% level. |
|
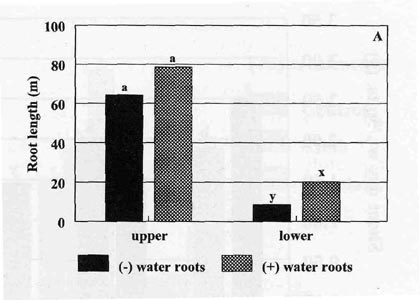
total root length for the upper part of the pots for
plants with or without water roots attached (Fig. 2A). However, the lower part
had significantly greater (P < 0.05) root length when the water root were
left intact at transplanting. Even though more roots were present in the bottom
of the pot, most of the roots were observed to be the original water roots
attached to the young transplant with very few new root initiations. Little or
no lateral root growth was observed from water roots while media roots exhibited
a highly branched and well distributed root system. Despite having a lower
metabolic activity than media roots (Caruso, 1999), the water roots were alive
and did potentially contribute to transplant development during the first two
weeks after transplanting.
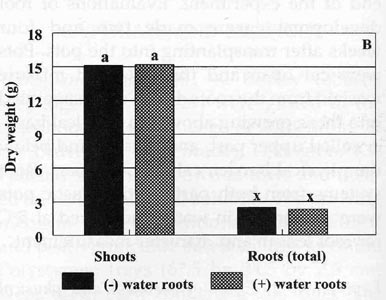
Four weeks after transplanting there were no significant
differences in the shoot or total root weight for plants transplanted with or
without intact water roots (Fig. 1B). Most of the water roots present were dark
brown in color and did not have the same healthy appearance as media roots. No
significant differences between treatments for total root length was measured
for upper, lower or total roots (Fig. 2B). In fact, most of the roots found in
the lower part of the pots, in both treatments, were media roots that grew
downward along the pot wall. These roots were counted as lower roots. The
slightly greater root length (approximately 600 cm) per plant with water roots
was basically due to the size of the original water roots attached to the
tobacco seedling at transplanting. Overall, these results indicated that intact
water roots on tobacco seedlings did very little to improve the establishment of
the transplants under the controlled greenhouse conditions of this experiment.
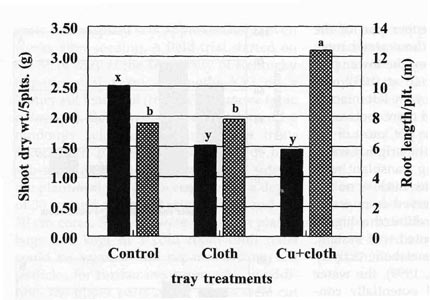
Experiment 2. Six weeks after seeding, tobacco transplants grown in trays treated with a physical barrier at the bottom of the float cell (Cloth) and trays with both chemical and physical barrier (Cu+Cloth) had significantly reduced shoot dry weight (P < 0.001) compared with control plants (Fig. 3).
 |
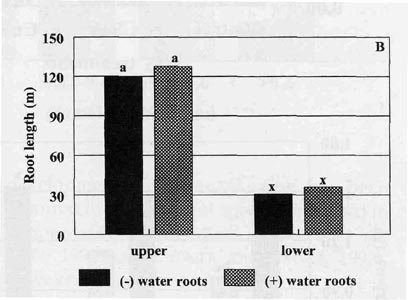 |
| Fig. 2. Total length of tobacco root systems 2 weeks (A) and 4 weeks (B) after transplanting to greenhouse pots. Upper = roots above the barrier; lower = roots below the barrier, (-) water roots = plants without water roots attached; (+) water roots = plants with water roots attached; bars represent mean values; means followed by the same letter are not significantly different from each other using t test (LSD) at 0.05% level. | |
The landscape fabric used to block the bottom of the
trays did not allow any root penetration into the nutrient solution. Seedlings
grown in untreated trays were much taller than seedlings in treated trays, but
these taller control plants were leggy with a long and thin stems.
Hormone imbalances created by the artificial root pruning may have mediated the
effect of restriction on shoot growth. Peterson et al. (1991) reported that root
restriction of tomato reduced concentrations of IAA in the leaves and
consequently reduced leaf area.
In the tobacco seedlings, mild toxicity (light brownish
color), confined mainly to the root tips, was observed when plants were removed
from the Cu+Cloth cells. Although the plants showed no significant difference in
root dry weight at the end of seedling development, the chemical pruning
treatment associated with root blockage (Cu+Cloth) produced a root ball with a
greater total root length compared to control tray plants (Fig. 3).
 |
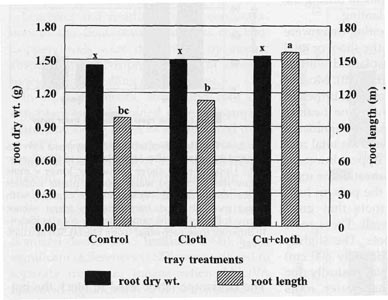 |
| Fig. 3. Shoot dry weight and root length accumulation of tobacco seedlings at transplanting time. Bars represent mean values; means followed by the same letter are not significantly different from each other using t test (LSD) at 0.05% level. | Fig. 4. Root length and dry weight of tobacco plants 4 weeks after transplanting to greenhouse pots. Bars represent mean values: means followed by the same letter are not significantly different from each other using t test (LSD) at 0.05% level. |
Four weeks after transplanting to greenhouse pots, there were no significant
differences in the total dry mass accumulation for roots produced by plants
grown in either treated or untreated tray cells
(Fig. 4). Nevertheless, the occurrence of a higher number of root branches due
to repeated pruning of tips as the roots interfaced with the interior cell tray
wall before transplanting may have stimulated the development of a longer root
system four weeks after transplanting. Cu+Cloth cells produced a plant with a
root length approximately 27% longer than Cloth cells (P < 0.005) and 60%
longer than Control cells (P < 0.001, Fig. 4).
The relative root distribution based on root diameter
showed that Cu+Cloth cells had significantly greater root length in all the
different diameter classes (Fig. 5). The fine roots (0.21 to 0.40 mm diameter
class) were the ones most frequent in all treatments. Approximately 65% of the
total root system of these plants occurred within the < 0.40 mm diameter
classes (Fig. 5). This high length accumulation of very fine roots (small
diameter classes) may have improved plant growth at four weeks after
transplanting. Seedlings from Cu+Cloth cells had more intact root tips (visual
observation). These root tips would provide a great advantage to transplant
establishment. The greater root length per unit mass (Fig. 3) also provides a
better root system for water and nutrient uptake that may aid in transplant
establishment in the field under adverse field conditions.
| Fig. 5 Distribution of tobacco roots according to different diameter classes 4 weeks after transplanting to greenhouse pots. Bars represent mean values; means followed by the same letter within diameter classes are not significantly different from each other using t test (LSD) at 0.05% level. | 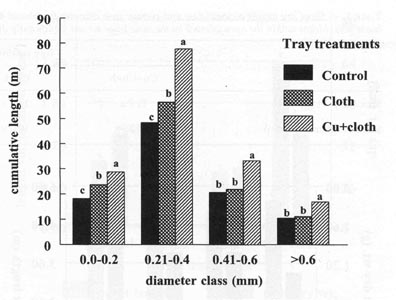 |
Morrison and Atkinson (1980) investigated the influence of different transplant size of hurley tobacco on survival and growth uniformity in the field. Seedling size did not significantly affect seedling survival, mature height or mature yield. At the end of four weeks, all the plants in this study had basically the same vegetative mass compared to the control plants (Table 1). However, at the transplant time, Cloth and the Cu+Cloth cells yielded a smaller transplant than the control cells. Increased shoot growth and development after transplanting has been reported in several plant species produced in copper-treated containers (Arnold et al., 1989, 1991 and Latimer and Baden, 1993), however others have reported no increase in shoot growth (Arnold and Young, 1991 and Geneve et al., 1995). It was readily observed that Cu+Cloth cells had shorter plants compared with non-treated plants. On the other hand, copper treated cells yielded very uniform plants with greater stem diameter (10.75 mm, P < 0.05) compared with the other treatments (Table 1).
Experiment 3. At
transplanting time (7 weeks after seeding), trays treated with (Cu+Cloth)
produced transplants with a greater shoot dry weight but the same length of
roots compared with other treatments (Fig. 6). This differed from the previous
experiment where Cu+Cloth produced smaller plants. In this experiment, seedlings
were clipped during development in the float system. Clipping reduced the
relative contribution of leaf mass to shoot weight, as a result the greater stem
diameter of Cu+Cloth treated transplants resulted in greater shoot weight for
this treatment.
Table 1. Shoot dry weight accumulation and average stem diameter for tobacco 4 weeks after transplanting to greenhouse pots. Means within the rows followed by the same letter are not significantly different at the LSD, 0.05% level.
| Tray treatments | |||
| Cu+Cloth | Cloth | Control | |
| Shoot dry wt (g) | 11.7 a | 11.0 a | 10.6 a |
| Stem av. diameter (mm) | 10.75 x | 10.0 y | 9.0 z |
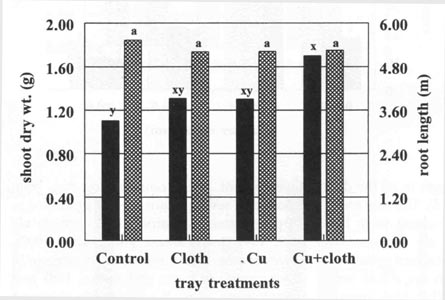 |
Fig. 6. Root length and shoot dry weight accumulation of tobacco seedlings at transplanting time. Bars represent mean values; means followed by the same letter are not significantly different from each other using t test (LSD) at 0.05% level. |
Fifty days after transplanting into the field, plants showed no differences in shoot development. Although there was a trend showing longer roots for plants grown in (Cu+Cloth) tray cells in the 0-15 cm depth, this difference was not significant due the large amount of variation among samples (data not shown). On the other hand, at the 15-30 cm depth, the (Cu+Cloth) provided plants with almost three times greater root system length compared to the control (Fig. 7). In addition, these plants had a root system with an extremely small diameter, in which approximately 50% of the roots had a diameter < 0.2 mm (Fig. 8).
| Fig.
7. Root length and dry weight (15-30 cm of depth) of tobacco plants 50
days after transplanting to the field. Bars represent mean values; means
followed by the same letter are not significantly different from each
other using t test (LSD) at 0.05% level.
|
|
| Fig. 8. Distribution of sampled tobacco roots (15-30 cm depth) according to different diameter classes. Bars represent mean values; means followed by the same letter within diameter classes are not significantly different from each other using t test (LSD) at 0.05% level. | 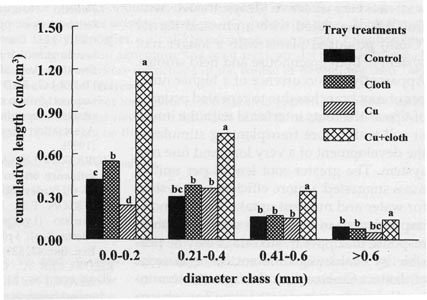 |
Plants from treated trays were 8 to 14 cm shorter than the control plants at 58 days after transplanting. At 68 days after transplanting 55% of the control plants were flowering compared to only 15% for treated plants. In contrast, at the end of the growing season (just before harvest), there were no differences in plant size or stem diameter (data not shown). The incidence of ground suckers was higher in plants grown in untreated trays (Control) than copper-treated trays (Table 2). Perhaps the greater root system formed after chemical and physical pruning modified the hormone balance and accumulation in the tobacco plant roots. Primarily auxins and cytokinins determine apical dominance in the plant. Accumulation and synthesis of these hormones in the root system, play a role in the growth of these lateral buds.
Table 2. Differences in tray treatments for percentage of ground suckers in the field. Means within the columns followed by the same letter are not significantly different at LSD, 0.05% level.
| Tray treatment | Number of ground suckers plant-1 | |||
| 0 | 1 | 2 | >3 | |
| Cu+Cloth | 14.3 a | 31.7 a | 41.3 a | 12.7 a |
| Cu | 6.7 a | 26.0 a | 40.0 ab | 27.3 b |
| Cloth | 11.7 a | 30.0 a | 22.0 b | 36.3 b |
| Control | 1.0 b | 13.3 b | 34.0 ab | 51.7 c |
Some concerns have been expressed about the potential
disposal of Cu contaminated float water resulting from the use of Cu(OH)2
on trays. The Cu concentration in water was monitored in float beds with
treated trays and found never to exceed 0.3 mg L-1. This is well below the drinking water standard of 1.0 mg
L-1 set by
the U.S. Environmental Protection Agency. The potential for environmental
contamination from the proper use of this particular
product (Spin Out) appeared to be relatively low.
|
CONCLUSIONS |
Two weeks after transplanting,
seedlings with intact water roots had a slight growth advantage, although there
was no new root initiation from the water roots. By four weeks after
transplanting the original water roots had turned brown, and no new root
initiation was observed. There was no growth advantage for seedlings planted
with water roots intact, suggesting that water roots did not serve a function in
the establishment of transplants under controlled greenhouse conditions.
There is enough evidence to suggest
that Cu+Cloth cell treatment has potential for tobacco transplant production in
the float system. Blocking just the bottom of the trays with fabric (Cloth) or
just painting the inner surface tray cells with the Cu-based latex was not very
effective. Trays treated with Cu(OH)2 associated with a physical
barrier (Cloth) produced plants with a longer root system in both greenhouse and
field studies. Apparently the occurrence of a higher number of root branches due
to repeated pruning of tips as the roots interfaced with the interior cell wall
before transplanting stimulated the development of a very long and fine root
system. The greater root length per unit of mass suggested a more efficient root
system for water and nutrient uptake. Furthermore, improvement in vegetative
shoot growth in response to copper treated trays may be possible by evaluating
different concentrations of distinct Cu-based products and determining the most
appropriated time for tobacco seedling roots to be exposed to the Cu-based inner
surface of the float tray cells. Tobacco transplants from copper-treated cells
must be evaluated under a wider range of environmental field conditions, and
grown for cured leaf yield evaluation.
|
REFERENCES |
ARNOLD
M.A., Timing, acclimation period, and cupric hydroxide concentration alter
growth responses to the Ohio
production system. J. Environ. Hort. 10,114-117
(1992).
ARNOLD M.A.,
AIRHART D.L., DAVIS W.E.: Cupric hydroxide-treated containers affect growth and
flowering of
annual and perennial bedding plants. J. Environ. Hort. 11,106-110
(1993).
ARNOLD M.A.,
STRUVE D.K.: Cupric carbonate controls green ash root morphology and root
growth. Hort. Sci.
24, 262-264 (1989).
ARNOLD M.A.,
YOUNG E.: CuCO3 - painted containers and root pruning affect apple
and green ash root growth
and cytokinin levels. Hort. Sci. 26, 242-244 (1991).
CARUSO L.V.:
Root function and development of float tobacco transplants. M. Sc. Thesis.
University of Kentucky,
USA (1999).
GENEVE R.L.,
BUXTON J.W., STANFFORD M.: Copper-hydroxide is an effective control of root
outgrowth in
plug-grown marigold seedlings subirrigated by capillary mats.
Proceedings of the Fourth National Symposium on
Stand Establishment, pp. 203-209
(1995)
HOYERT J.H.:
Growing and transplanting tobacco seedlings with intact root systems. Md. Agric.
Exp. Stn. Coll. Park.
Misc. Public. No. 944, 29 (1979).
LATIMER J.G.,
BADEN S.A.: Spin Out enhances subsequent growth of tomato, but not pepper,
transplants. Proceedings
of Southern Nurserymen's Association Research
Conference 38, 120-123 (1993).
MORRISON J.E.,
ATKINSON W.O., Seedling size influence on survival and growth uniformity of
no-till burley tobacco.
Tob. Sci. 24, 93-94 (1980).
PETERSON T.A.,
REINSEL M.D., KRIZEK D.T.: Tomato (Lycopersicon esculentum Mill., cv.
"Better Bush") plant
response to root restriction. J. Exp. Bot.
42,1233-1240 (1991).
RIEDACKER A.,
DEXHEIMER J., TAKAVOL R., ALAOUI H.: Modifications experimentales de la
morphogenese te
des tropismes dans le systeme racinaire de heunes chenes. Can.
J. of Bot. 60, 765-778 (1982).
SAS Institute. Inc. SAS/STAT User's Guide, Version 6, Cary, NC, USA (1996).
SMITH W.D.: Transplant production. North Carolina Burley Production Guide. North Carolina State University, USA (1998).
WIGHTMAN F.,
THIMANN K.V. Hormonal factors controlling the initiation and development of
lateral roots. I. Sources
of primordia-inducing substances in the primary root
of pea seedlings. Physiol. Plant. 49,13-20 (1980).