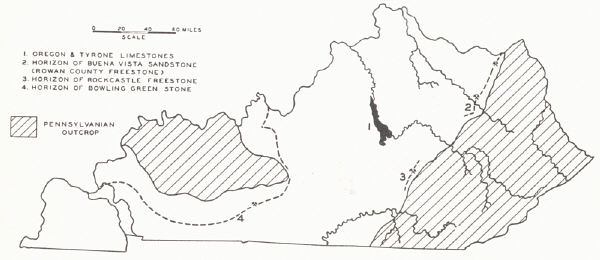
BUILDING STONE1
Five building stones of merit are commonly recognized in the state. Other limestones and sandstones for foundation work and general construction purposes are widespread. The demand for building stone of all grades, though, has suffered from competition with concrete and concrete products so that the industry is less flourishing than formerly.
 |
| FIG. 41. Building stone in Kentucky |
(1) The Oregon limestone outcrops in the Kentucky River region of the Inner Blue Grass. It is a fine-grained grey to cream-colored dolomitic limestone of Lowville age, 15 to 25 feet thick and known as the Kentucky River marble. Quarries were formerly operated in a number of localities in Fayette County. Boone's monument and the column of the old State House at Frankfort were built of this material, as was also the shaft of Clay's monument in Lexington. It is a high grade stone but has only been used locally.
(2) The Tyrone (Lower Birdseye) limestone likewise is found in the Kentucky River region of the Inner Blue Grass. It is a dense dove to grey limestone 90 feet thick with facets of coarsely crystalline calcite scattered through it. The rock weathers white, and it is the darker facets of calcite that give it the "birdseye" character. It has been widely used in central Kentucky for building purposes, including residences, the old Capitol Hotel, the old State House and penitentiary walls at Frankfort, and some of the older distilleries along the Kentucky River. In many of these the rock was sawed into blocks. It is a high grade building stone.
(3) The Rowan County sandstone (freestone)—the Buena Vista sandstone member of the lower Waverly (Cuyahoga)—is quarried at Farmers, Freestone, and Bluestone, Rowan County. It is a fine-grained grey sandstone 18 feet thick remarkably even-bedded, and with some beds up to 40 inches thick. It has been the most widely used sandsto4e in the state for various building purposes, flagging, curbing, etc. The Buena Vista sandstone member is known as far south as Powell County where it has a thickness of five feet. To the north it has been quarried near Tannery in Lewis County and at Buena Vista, Ohio, on the Ohio River, from where much of the product was shipped to Cincinnati.
(4) The Rockcastle freestone is quarried in Rockcastle County by the Kentucky Freestone Company, which began operations in 1896. Butts (1922) identified it as the Wildie sandstone (p. 75). A large part of the sandstone is sold as sawed stone and is well adapted to cut stone. It has been widely used outside the state. It occupies about the same stratigraphic position as the Big Injun oil and gas sand of the Eastern Coal Field.
(5) The Bowling Green limestone is the outstanding building stone of the state. It is a massive oolitic limestone of the Lower Chester2 (Renault¬-Paint Creek). It is similar in character, though of different age, to the Bedford limestone of Indiana. Any dinginess as a result of occluded pe¬troleum when freshly quarried soon disappears as the stone bleaches. It wears well. Hearth stones erected more than a century ago are intact with little or no evidence of disintegration. Buildings almost a century old in Bowling Green still retain the original tool marks. Among buildings made from it may be mentioned: several buildings of Western State Teachers College, Bowling Green, Kentucky; the Carnegie Library, Nashville, Tennessee; Chamber of Commerce Building, Atlanta, Georgia; the Good Samaritan Hospital, Lexington, Kentucky; United States Government buildings in Jackson, Tennessee, Carmi, Illinois, Jackson, Mississippi, Gulfport, Mississippi, Pensacola, Florida, and Jacksonville, Florida.
The undivided Renault-Paint Creek outcrops in a belt along the Dripping Springs Escarpment along the eastern and southeastern edge of the Western Coal Field. From Christian County westward the limestone succession is interrupted by the introduction of the Bethel sandstone, in Breckinridge County and northward by the equivalent Sample sandstone. To what extent the desirable features of texture and bedding are developed throughout this belt has not been adequately looked into.
Regional Distribution
From a regional point of view the availability of building stones may be summarized as follows:
Central Blue Grass.—The Oregon and Tyrone limestones are widespread. A number of the formations constituting the Lexington limestone, particularly the Benson, have been used for the building of homes and other purposes. It is a coarsely crystalline limestone of considerable merit. The Cynthiana is a more shaly formation, but locally, as at Cynthiana and in Nicholas County, Kentucky, an upper member, the Nicholas limestone, is massive and quarried. The so-called River Quarry beds at Cincinnati and southeast to Moscow along the Ohio River correlate with this same member. The Fairmount in the northernmost Blue Grass and the heavy-bedded Louisville and Jeffersonville limestones of Jefferson, Bullitt, and Nelson counties are suited for building and other purposes. The Laurel dolomite in this vicinity may also be used.
The Knobs.—In the northern part of the eastern belt of Knobs in the vicinity of the Ohio River the Berea sandstone is a highly desirable sandstone for structural purposes and is quarried in Lewis County and vicinity. This formation thins out southward and is not recognized south of the Ashland-Louisville branch of the Chesapeake & Ohio Railroad. The Rowan County and Rockcastle freestones have been discussed.
Cumberland Plateau.—The outcrop of the Rowan County and Rockcastle freestone is marginal to the Knobs and Plateau. These formations have been described. Taking the area as a whole there are various Pennsylvanian sandstones which have a limited use locally. In the vicinity of Paintsville, Johnson County, a local sandstone was used in the erection of Mayo College and the Mayo Memorial Church. To this unit Richardson (1923) applied the name Paintsville sandstone.
Pine Mountain.—Mississippian limestones outcrop again on the upthrow side of the Pine Mountain thrust fault.
Middlesboro Basin.—As in the Cumberland Plateau there are Pennsylvanian sandstone formations available for local usage.
Pennyroyal and Mammoth Cave Plateaus.—The Bowling Green building stone is outstanding and has been discussed. In the Mammoth Cave Plateau country a number of Chester sandstones and limestones outcrop. These, though not noteworthy, have been used in some places.
Western Coal Field.—There are various sandstone formations of Pennsylvanian age but in general they are little used. In Hopkins County the locally much thickened Madisonville limestone is quarried.
Jackson Purchase.—The formations of this region are all unconsolidated sediments of the Cretaceous, Tertiary, and Quaternary.
LIMESTONE AND DOLOMITE
Limestone (including dolomite) is a mineral resource of many uses, such as building stone, general construction, road metal, refining beet sugar, a flux in metallurgical work, a source of agricultural lime, the making of Portland cement and various cement products, alkalies, dolomite refractories, calcium carbide, and rock wool.
The materials are widespread in the state. Limestone forms a very large part of the outcropping Ordovician section and also of the Meramec and Chester. It occurs in considerable thickness in the Middle Silurian and Middle Devonian on the northwest side of the Arch as the Louisville, Jeffersonville, and Sellersburg formations. There are two great limestone regions in the state—the Blue Grass and the Mississippian Plateaus. It is present in very limited quantities in the Western and Eastern coal fields and not at all in the Purchase. In Pine Mountain and again in Cumberland Mountain it reappears in surface outcrop in the Pine Mountain overthrust.
Within the state limestone is used for building stone, road metal, railroad ballast, lime, agricultural limestone, portland cement, and rock wool.
PORTLAND CEMENT
Portland cement is the most used type of hydraulic cement. It is made by the burning (clinkering) of a finely ground artificial mixture of limestone and silica and alumina (clay or shale or sometimes furnace slag). The mixture calls for about 75 per cent CaCO3 and about 20 per cent silica, alumina, and ferric oxide. Up to 2 per cent magnesium carbonate and 2 to 3 per cent pyrite is allowed in limestones used. In the shale or clay, silica should be two and a half to four times the alumina, and ferric oxide should not exceed alumina, preferably should be about 1:3. Some impure limestones approximate the desired proportions (cement rock) and are used directly for the manufacture of natural cement. Such was the case with the Silver Creek member of the Sellersburg limestone in southern Indiana and at Louisville. The use of various cement products, such as building block, concrete brick, and roofing tile, is on the increase.
Portland cement is manufactured at Kosmosdale about 20 miles south of Louisville on the Ohio River. Materials used are limestone of the Fredonia member of the Ste. Genevieve from near Brandenburg in Meade County and alluvial clays of the Ohio River floodplain. Though not used, the New Providence and Rosewood shales are available in the vicinity. In the manufacture of this product competing transportation facilities constitute an important item for the product is bulky.
Available materials are rather widespread.
Limestone.—The outstanding Ordovician limestone in so far as purity and availability are concerned is the Tyrone which outcrops over a wide area in the Kentucky River region of the central Blue Grass. The associated Camp Nelson and Oregon limestones are high in magnesia. An occasional bed of the Camp Nelson type high in magnesia occurs within the Tyrone.
The Lexington and Perryville limestones outcrop over a wide area in the central Blue Grass and these limestones are relatively low in magnesia. The Woodburn particularly, and sometimes other beds, show a high percent of phosphorus. There is considerable associated shale. The Benson seems best adapted to the purpose.
The quarry rock of the upper Cynthiana (Nicholas) in Nicholas, Pendleton, and Harrison counties seems desirable according to the few analyses available. The Fairmount (Hill Quarry beds) at Cincinnati (and by inference also for that vicinity in northern Kentucky) shows a favorable content of magnesia. No analyses are available of the quarry rock of the Mt. Auburn of Fleming County and vicinity. Other Ordovician limestones are either impure or associated with a large amount of shale.
The Silurian Brassfield, Laurel, and Louisville limestones are high in MgCO3. The Mid-Devonian Jeffersonville limestone in Jefferson County and vicinity has a suitable composition, but in its typical development is known only in a belt along the Ohio River in Jefferson and Oldham counties. The Silver Creek limestone (lower Sellersburg (Hamilton), known as the hydraulic limestone or cement bed) was formerly extensively utilized for the manufacture of natural cement in southern Indiana (Siebenthal, 1901) and to a small extent at Louisville. It has a thickness not in excess of 10 feet and is not known in outcrop except in a narrow belt along the Ohio River near Louisville.
Limestone constitutes a very large part of the Meramec and Chester section and much of it is a pure rock low in magnesia. Chert is abundant in much of the St. Louis but not all. The Warsaw rather characteristically is geodiferous, but this feature again is limited to certain zones and areas. Much of the formation is pure crystalline limestone. The Ste. Genevieve and Lower Chester forms an extensive outcrop of good limestone and is most characteristically developed along the Dripping Springs Escarpment in the west and along the Pottsville Escarpment in the east. To the north along this Escarpment in eastern Kentucky the Ste. Genevieve becomes sandy and in parts of Lewis County the entire limestone section is unconformably out. As pointed out, limestone of the Fredonia member of the Ste. Genevieve is used at Kosmosdale. It would seem that the Bowling Green building stone (Gasper) would be desirable.
Limestones of Pennsylvanian age are too limited in extent and thickness to be of commercial importance. In some instances, as at Ironton, Ohio, high grade limestone (Maxville = Big Lime of eastern Kentucky) has been mined underground in the region of Pennsylvanian outcrop. The chief objection to shipping limestone any distance is the weight, 44 per cent of which is lost in the burning.
Shales and Clays.—Available clays and shales include the various formations listed under clays elsewhere. These include:
(1) Eden shale of the Blue Grass.
(2) Crab Orchard shale of the eastern edge of the Blue Grass and Knobs.
(3) Ohio black shale. It has been suggested that this bituminous matter present is an asset rather than otherwise, constituting an additional source of fuel in the clinkering.
(4) New Providence shale (widespread in the Knobs).
(5) Shales of the Upper Chester (Leitchfield-Buffalo Wallow, and Pennington).
(6) Various shales of the Pennsylvanian.
(7) The clays of the Tertiary in the Jackson Purchase region.
(8) Residual clays from the weathering of limestone and shale (now often used in the manufacture of brick), and alluvial clays along the major streams. The latter are used by the Kosmosdale plant.
The problem of fuel involves little difficulty, though the limestone regions of the state are not the coal-producing regions. However, the abundant supply of coal from both the Eastern and Western fields to¬gether with rather widespread petroleum and natural gas production gives an abundant available supply of fuels within a reasonable distance of any plant or available supply of the necessary raw materials.
LIME
Lime is made by burning limestone or dolomite at a temperature high enough to drive off the carbon dioxide. Commercial limes seldom contain ail excess of five per cent silica, ferric oxide, and alumina. Variation from high calcium to high magnesium lime is a matter of the use to which it will be put. Availability of suitable limestone has been indicated on the pre-ceding pages. The main commercial use is in various chemical industries, for building purposes, dead burned dolomite, and for agricultural purposes.
Only one operation was listed in Kentucky in 1937 (Minerals Yearbook, 1938), that at Pine Hill in Rockcastle County. Lime is locally burned for agricultural purposes at many places in the state.
ROCK WOOL
Rock wool is an insulating material of increasing importance, commonly manufactured from argillaceous limestone or purer limestone in combination with shale or sandstone. It is also made from slag (slag wool) and other materials. It is a substance composed chiefly of the silicates of calcium and aluminum and composed of interlacing fibers with the general appearance of loose cotton or wool. Lamar and Willman (1934) have used the term woolrock for rock which will yield a rock wool without the addition of other materials, and sub-woolrock for those which require additional material. The process of manufacture is one of fusing the rock and then blowing the liquid into fibers by a strong jet of steam. There seems to be a very wide range in possible composition, and it has been shown by Lamar and Willman (1934) that the presence of neither MgO nor A1203 is essential. A very essential feature is that the fused materials have a prolonged period of viscosity so that the "shot" will be pulled out into threads (Thornbury, 1938).
The mineral wool industry was introduced in this country in Indiana in 1897 and that state is the main seat of the industry with sixteen operating plants (1937). One makes a slag wool, nine use the Mississinewa calcareous shale (Niagaran), and the other six use the following (Thornbury, 1938, p. 167):
Salem limestone and Borden shale Devonian limestone and top soil Harrodsburg limestone and Chester sandstone Salem limestone and clay River gravel and limestone Chester (?) limestone and shale
These are listed here not only to give a general idea of the raw materials, but because outcropping formations in Indiana are much like those in Kentucky. Lamar and Willman made an extensive study of the composition ranges of suitable rock and concluded (1934, p. 197-198):
". . . any naturally occurring mixture of shale, sandstone, and dolomite or limestone, which contains from 20 to 30 per cent carbon dioxide and which does not contain excessive amounts of impurities, is either suitable, from the standpoint of composition, for the production of rock wool, or can be made suitable by the addition of very small amounts of a rock containing a high percentage of the deficient component. This easily determined carbon dioxide content is therefore a most useful criterion by which to judge the suitability of samples for rock wool production."
Two plants are operating in Kentucky: (a) the Kentucky Stone Company at Mullins, Rockcastle County, and (b) the Ohio Valley Rock Asphalt Company at Summit, Hardin County. Limestone in combination with shale, sandstone, and slag is used. There is no problem as to the more than adequate availability of materials in the limestone regions of the state, the only problem being one of market and development.
| TESTS OF MISCELLANEOUS KENTUCKY SHALES AND
CLAYS From H. Ries, 1922 |
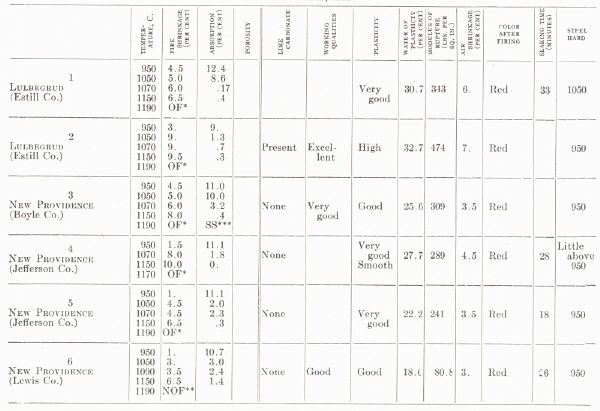 |
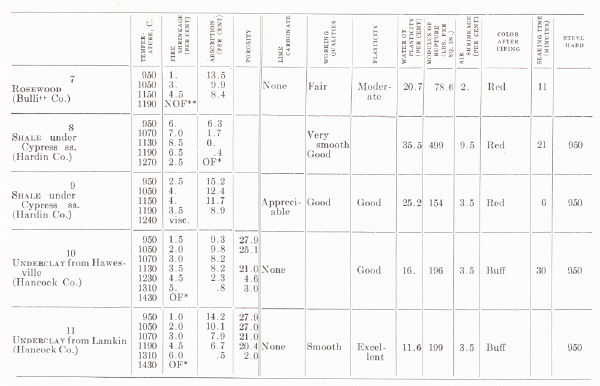 |
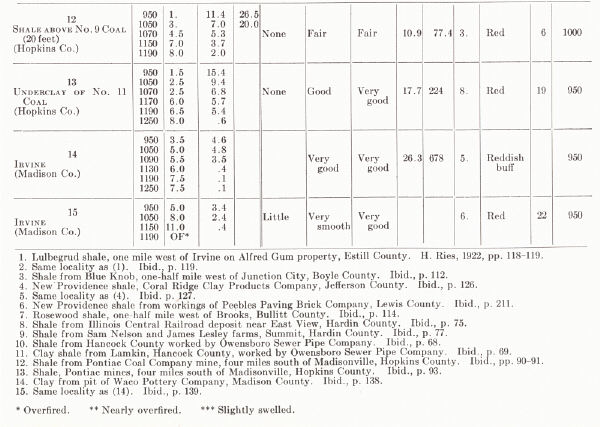 |
AGRICULTURAL LIMESTONE AND LIME
Ground limestone or lime is extensively applied to soils for the neutralizing of soil acids, but it is also otherwise beneficial. Except for the areas of Pennsylvanian outcrop and the Purchase region there is a large available supply. The occasional limestones and marls of the coal fields are locally important. Since MgC03 has greater neutralizing value than CaC03, dolomitic limestones are not inferior.
ROAD MATERIALS OTHER THAN ROCK ASPHALT
An abundant supply of suitable limestone is found in the Ordovician3 rocks of the Blue Grass, in the Louisville, Jeffersonville, and Sellersburg limestones of the northwestern Blue Grass border, and in the limestones of the Meramec and Chester of the Mississippian Plateaus. This is not the case in the Eastern Coal Field, Western Coal Field, and Purchase region. In general, small quarries have been opened near the road building operation. The Mississippian limestones brought up in Pine and Cumberland mountains by the Pine Mountain overthrust take care of the border counties in the southeast. The Madisonville limestone attains an unusual thickness at Madisonville in Hopkins County and is quarried for this purpose. Farther west gravels of the Ohio, Cumberland, and Tennessee rivers and their tributaries are used. Those of the Ohio River, which include glacial material, chert from the St. Louis limestone, and quartz pebbles from the lower Pottsville seem best. Considerable trouble has been encountered with concrete made from the cherty gravels of the Tennessee River. These gravels include not only chert from the St. Louis and Fort Payne formations, which occur also in Cumberland River gravels, but also chert from several Devonian formations of western Tennessee. These latter seem less stable and react in some manner in the concrete. Whatever the precise cause may be, the concrete does not stand up well.
ROCK PHOSPHATE
An important factor in the fertility of Inner Blue Grass soils has long been recognized as the unusually high phosphorus content. The soils are residual, derived from Mid-Ordovician limestones, principally from those of the Lexington limestone, and the best is that from the Woodburn formation. In 1904 Miller found deposits of leached phosphate rock two miles east of Wallace in Woodford County, which ran as high as 72 per cent tricalcium phosphate, and he called attention to its commercial possibilities. In 1915 the discovery of similar material on the farm of H. L. Martin near Wallace led to its commercial use by the Central Kentucky Phosphate Company.
Investigations by Miller, Phalen, and Foerste have indicated the geological situation. The occurrence of the phosphate is controlled by the distribution of the Woodburn limestone, which is highly phosphatic. Rich bands alternate with those containing less or little phosphate. They often show cross-bedding and commonly have something of an "oolitic" appearance because of the abundance of the minute fossil snail Cyclora minuta. A similar association has been observed locally in the upper Benson and lower beds of the Cynthiana. The shells of this species have been recognized as having some relationship to the occurrence of the phosphate, for the organism is represented by internal moulds of phosphate of lime.
There is some question as to the original source of the phosphate in the limestone.4 This is discussed in some detail by Smith and Whitlatch (1940) in their report on the phosphate resources of Tennessee. The source has been regarded as the phosphatic shells of marine organisms and Miller (1896) emphasized the great abundance of Cyclora minuta. These shells are consistently and abundantly present in the phosphatic layers and the shell was presumed to have originally been phosphatic. Granting this to be true, though, the great abundance of these shells may have only been a response to an environment of waters unusually rich in phosphorus, which is an item still to be explained. However, the occurrence of the phosphate in association with Cyclora, which is the common and characteristic association, is as the internal mould, and the shell is usually missing. The phosphate occurs also within zooecial tubes of bryozoa and similarly in other shells as small nodules and as a partial or complete replacement of shell material. Smith and Whitlatch (1940, p. 41) concluded:
"that the phosphatic Ordovician limestones of Middle Tennessee did not receive any considerable part of their phosphatic content in the form of remains of marine animals that secreted phosphatic shells. The only other possible explanation is that phosphate in the sea water was precipitated on the sea bottom by some bio-chemical or physiochemical agency, along with the calcareous shells and remains of the animals living in the sea, and that, while the sediments were still soft and unconsolidated, the phosphate thus precipitated partially replaced some of the calcareous fossils."
The problem of the original source of the phosphorus in these seas and of the condition of an unusual abundance of it is still unanswered.
Concentration into deposits of commercial value has been a matter of (a) residual concentration by the selective leaching of the limestone (main process according to Phalen, 1917), and (b) replacement of the underlying limestone (Brannon and Benson) by phosphate removed in solution (re-garded as the principal process by Miller, 1919). It is only a matter of relative importance, for both processes are involved. The deposits occur along the uneven surface of the limestone. Solution along joints has opened the "cutters" leaving "horses" in, between. For a discussion of the problem see Phalen (1917), Miller (1913,1919), Foerste (1913b), Twenhofel (1931), Clarke (1924), Hook (1914), and Smith and Whitlatch (1940).
Analyses of selected samples from lump phosphate range as high as about 80 per cent tricalcium phosphate and more than half show over 70 per cent. Favorable areas for prospecting have been regarded as: (a) those in which the Woodburn outcrops or where the residual soil has been derived from it, and (b) those where the upland is comparatively level and in which, as a result, residual concentration was favored.
SAND AND GRAVEL
Glass Sands.—Glass sand has been produced on a small scale, but figures are unavailable, for they have been combined with those of other states in reports of the Minerals Yearbook, in part to avoid revealing confidential information. Several types of deposits either in use or available were described by Richardson (1920).
(a) The lower Pottsville is quarried at Olive Hill, Carter County, by the General Refractories Company for a molding sand. The lower 15 feet of the quarry is a friable white to faintly yellowish white sandstone. The upper 20 feet is iron stained from surface seepage but can be washed. A sandstone of about the same stratigraphic position is used by the Camp Glass Company from two quarries at Tygart in the same county.
(b) River sands (Big Sandy River) from near Zelda, Lawrence County, were formerly used by the Covington Glass Company, and it is reported that Ohio River sands from near Sandy City (between Ashland and Catlettsburg) were similarly used.
(c) A large deposit of high grade glass sand is worked at Tip Top, Hardin County, by the Kentucky Silica Company of Louisville. It is a soft white sandstone of fluviatile origin 400 feet above the level of the Ohio River and occupies limestone sinks in the St. Louis limestone in the upland behind Muldraughs Hill. It was referred to the Ohio River formation by Burchard (1907a) and mistakenly to the Big Clifty (Cypress) by Richardson (1920). It is apparently a late Tertiary sandstone antedating the cutting of the Ohio River trench and is presumably reworked Cypress sandstone and perhaps others. The lower 25 feet is pure white, the upper part more or less iron stained from surface seepage. The upper sand is used for molding sand, in brick factories, for plaster, construction work, etc. Two grades of glass sand are sold, and the better (No. 1) is a high grade sand "suited for the manufacture of the best grades of glass . . ." (Richardson, 1920, p. 94).
(d) A quarry was opened about 1890 at East View, Hardin County, in what is probably the Cypress sandstone, and it is a high grade glass sand. The quantity is almost inexhaustable and it is shipped to New Albany, Indiana, and several places in West Virginia for the manufacture of glass and elsewhere for molding sand and other purposes. There are 40 to 45 feet of white sand and a few feet iron stained above.
(e) Sand for glass manufacture has been shipped from pits at Marion, Crittenden County, to Evansville, Indiana. There is a thickness of 30 to 40 feet, but the identity of the formation is not known other than it is Chester.
In his study of available glass sands Richardson (1920) recommended as high grade glass sands: (a) the lower Pottsville (developed at Olive Hill and Tygart in Carter County); (b) sands of the Tip Top type which, though, are of local occurrence, and (c) the Cypress of western Kentucky. Analyses given by the same author were made from unwashed sands. Washing will reduce the percentage of alumina and iron and correspondingly increase the percentage of silica. The Cypress sandstone ("Big Clifty") was examined at a number of localities. At Princeton, Caldwell County, 30 feet of pure white friable sandstone was referred to as the finest glass sand in the state.
Glass sands of fair quality which require washing and screening occur along the Ohio, Big Sandy, and other rivers. Many of these are used for construction purposes.
Other sandstones sampled, some of which are used for building purposes (Homewood, Mahoning, Buena Vista, etc.) may be crushed to a fairly white fine grained sand but are too rich in alumina for the best glass.
Richardson found an adequate supply of high grade glass sand and associated with it in Kentucky an adequate supply of pure limestone, fluorspar, and natural gas. The study, though, was cursory and sampling was sporadic with little detailed differentiation of stratigraphic units.
Molding Sand.—These sands must possess: (a) permeability high enough to permit ready escape of gases, (b) cohesiveness (strength) due to the presence of a film of clay, limonite or other bonding material, (c) fineness and uniformity of grain, and (d) refractoriness (feldspar and mica are un-desirable). Molding sands from several sources have been used in Kentucky for years though the production is not large. It is mainly in the vicinity of the larger industrial centers such as Ashland, Louisville, and Cincinnati.
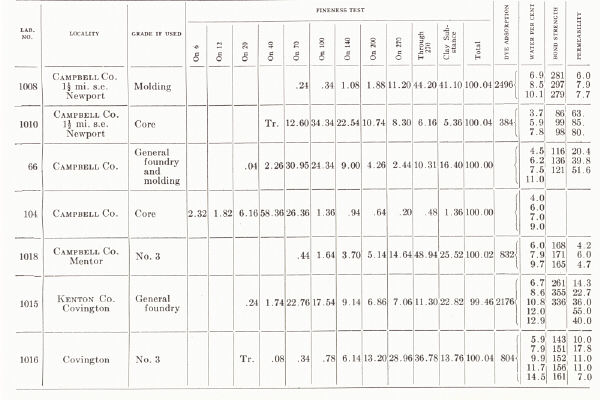
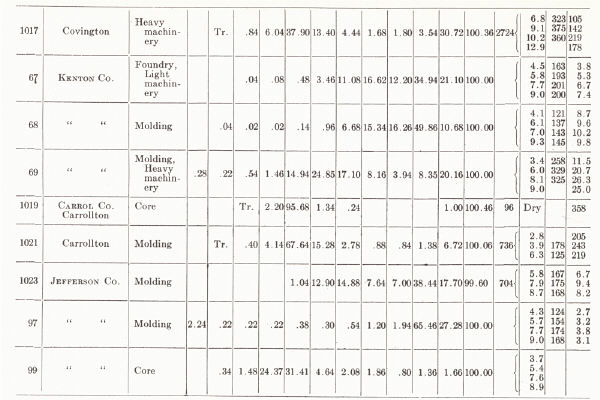
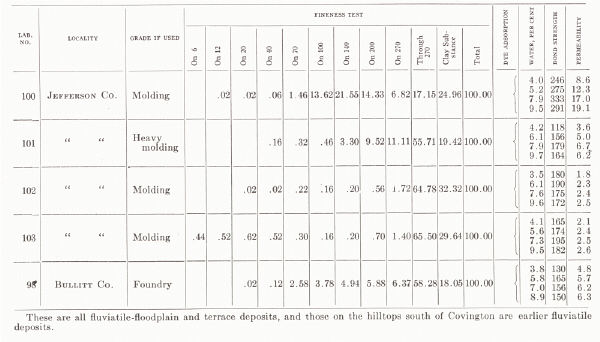
(a) Sand has been taken on a large scale from the pits at Tip Top and vicinity, Hardin County, and sold as No. 1 (floor and facing sand for brick plants) and No. 4 (a heavy molding sand for heavy machinery, etc.). The deposit operated at Tip Top by the Kentucky Silica Company is 50 feet thick, and the lower 25 feet is sold mainly as a glass sand.
(b) Molding sand is obtained in considerable quantities from deposits on the upland just south of Covington in the vicinity of Devou Park and Park Hills. These are fluviatile deposits, possibly related to the preceding and are worked for various grades of sand, including molding sand for light and heavy machinery, aluminum, and brass.
(c) Alluvial sands of the floodplain and terraces of the Ohio and other larger rivers are available and are used in Boyd and Greenup counties. A deposit at Russell (Greenup County) is referred to by Richardson (1927a) as one of the best core sands in the state. A deposit two miles north of Ashland has been used for brass foundry work. Another two miles southwest of Ashland has been used for heavy machinery, core sand, and common molding sand, and is described as originating from glacial ponding (Richardson, 1927a).
They are worked around Cincinnati in Kenton and Campbell counties. A large deposit at Mentor produces five grades listed as (1) open core, (2) open brass, (3) grey iron and malleable iron castings, (4) aluminum and light castings, and (e) heavy loam for machinery sand.
Similar deposits are worked around Louisville in Jefferson and Bullitt counties. Sand is pumped from the river bed at Kincaid Island in McCracken County and sold as molding sand.
(d) Cretaceous sands of fluviatile origin in the edge of the Purchase region have been worked as molding and core sand.
(e) The Cypress sandstone (Big Clifty) is worked at East View in Hardin County and tests elsewhere indicate that it is suitable for core sand.
(f) Lower Pottsville sandstones have been extensively worked at Tygart in Carter County and shipped to several states. Similar sandstones worked by the General Refractories at Olive Hill are used for floor and facing sand in brick plants. Sandstones at about this same stratigraphic level in Boyd County have been used for cores and general molding.
From the above notes and from sampling and tests made by Richardson, a general availability of molding sands is indicated. From experience in Carter and Boyd counties it is apparent that the lower Pottsville and perhaps other Pennsylvanian sandstones should provide a large supply not only in the eastern area but also the western. The Cypress sandstone is widespread along the margin of the Western Coal Field. Other Chester sandstones are of similar character, and some of them may be available. Deposits of the Tip Top (Hardin County) and Devou Park (Kenton County) type will necessarily be of restricted geographic occurrence. Floodplain and terrace sands are widespread along the larger streams. The terrace sands are widespread along those streams affected by the Pleistocene glaciation, the Ohio River and the lower course of its tributaries.
Sand and Gravel for Miscellaneous Purposes.—Sand and gravel used for miscellaneous purposes is abundantly available in: (a) streams, particularly those draining the regions of sandstone outcrop (Chester and Pennsylvanian sandstones); (b) the friable sandstones of the Chester and Pennsylvanian which are worked in a number of places; (c) deposits of the Tip Top and Devou Park type mentioned above.
Gravel is particularly available along streams draining the area of lower Pottsville conglomerates (Rockcastle and Corbin of eastern Kentucky and the Caseyville of western Kentucky). Another common source of gravel is the chert of the St. Louis and to a lesser extent the Ste. Genevieve, Fort Payne, and other chert-bearing formations. A third source is the glacial outwash. This is found, of necessity, only along streams which drained southward and westward from the ice sheets of the Pleistocene.
| KENTUCKY MOLDING SAND From C. H. Richardson, 1927, pp. 58-61 |
 |
 |
 |
COMMERCIAL BRINES
Natural brines have been much sought for throughout historic time as a source for common salt. The importance of the American salt licks in colonial times is indicated by acts of Congress and legislatures to protect and encourage the development of salt spring properties and to prevent their monopoly. Later, brines came into extensive use as a source of pharmaceutical and industrial chemicals. Among the more common products are sodium, calcium, and magnesium chloride, chlorine, soda ash, caustic soda, metallic magnesium, bromine, and iodine.
The early exploitation of salt licks in Kentucky is described by Clark (1938). The first lick to attract attention was Big Bone Lick in Boone County. Other important licks included Upper and Lower Blue Lick in Nicholas County, Drennon Lick in northern Henry County, Mann Lick and Bullitt Lick near the mouth of Salt River and near the Jefferson-Bullitt county line. Numerous licks were known in Clay County. The salt works on Goose Creek is well known. Salt water along with gas from a number of wells was the basis of a flourishing salt industry in the vicinity of Cloverport, Breckinridge County, in the early days (Miller, 1919).
An early salt industry was developed along the Kanawha River in West Virginia. It later came into disastrous competition with the stronger brines discovered around Pomeroy, Ohio. Figures by Price et al. (1937a, p. 12, quoting Lippincott) give the concentration of these early known brines in terms of gallons of brine required for one bushel of salt.
Blue Lick, Kentucky—1000 gallons per bushel.
Kanawha Valley—75 gallons per bushel.
Muskingum, Ohio—50 gallons per bushel.
Zanesville, Ohio—95 gallons per bushel.
Shawneetown, Illinois—120-180 gallons per bushel.
Boone's Lick, Missouri—450 gallons per bushel.
In Kentucky operations, ordinarily, salt makers estimated one bushel of salt (50 pounds) from 400 gallons of water (Clark, 1938). As indicated, the Blue Lick water is relatively dilute.
No investigation has been made of Kentucky brines for industrial purposes. As a result, little is known of their availability in suitable concentration and volume. Reports on such investigations have been published by both the Ohio and West Virginia geological surveys from which some inferences regarding Kentucky may be drawn.
Ohio Brines (Stout et al., 1932).—The more conspicuous brine-bearing zones include:
(a) The First Salt Sand (Massillon sandstone—above the horizon of the Corbin conglomerate in Kentucky) of the Pottsville was a source of brine for some of the early salt works along the Hocking River and at Pomeroy.
(b) The Lower Salt Sand brines were the first utilized in Ohio and were used to some extent in the Pomeroy field. This is the Sharon conglomerate and in Ohio is referred to as the Maxton. The usage of the term is not in harmony with that in West Virginia and Kentucky where the Maxton (or Maxon) is a sandstome member of the Pennington = Mauch Chunk.
(c) Brines from the Big Injun (upper Waverly) have for many years been the main reliance for salt making at Pomeroy where the main products are sodium chloride, calcium chloride, and bromine. Brines from the Keener sand are similar to those of the Big Injun. In Kentucky the Big Injun sand is commonly not divided into the Keener, Big Injun, and Squaw. The Berea was a commercial producer at Pomeroy in the early days of the salt industry.
(d) The Oriskany (First Water of the Big Lime) is regarded as available for industrial use.
(e) The Newburg (Second or Big Water of the Big Lime) is the chief water-bearing zone of the deeper rocks. It is widespread in eastern Ohio, and is recommended for industrial use as of great volume and high concentration. This zone seems to have essentially the same stratigraphic position as the upper pay of the Irvine field, Estill County, Kentucky.
(f) The St. Peter brines were formerly used for salt making at Cincinnati and "offer much material for the chemical industries" (Stout et al., 1932).
The authors found a rather consistent increase in concentration with depth. Occasionally iodine occurs in appreciable amounts in the deeper seated waters.
West Virginia Brines.—An outline of available brines in West Virginia was published by Price et al. (1937a). The greatest brine producers are the several Salt Sands. These are regarded as offering the greatest possibility for commercialization because of their great extent and volume, and the relatively shallow depth at which they are encountered. They are usually more concentrated in the southern part of the state. Analyses given show a maximum of 158,900 parts per million, with 100,000 parts common. The Maxton is not so consistently productive, but where encountered the brines are abundant. A maximum concentration of 167,000 parts per million is listed. The Big Injun (including Keener, Big Injun, and Squaw) produces large volumes of concentrated brine in some localities. Analyses show up to 187,000 parts per million and an average of 92,470. The Oriskany shows a higher concentration than the shallower sands.
Kentucky Brines.5—Little is known of the commercial possibilities of Kentucky brines, for few analyses have been made. As indicated above, brines from the St. Peter were formerly used at Cincinnati. Analyses from five "St. Peter" wells in Kentucky, though, show them to be quite dilute as compared with commercial brines, and the Old Crow Distillery well in Frankfort even more so.
*Lebanon Junction6 (1600')—16,600 parts per million.
Old 76 Distillery, Newport7— (1250')—11,680 parts per million.
Fisherville well, Jefferson County8—11,407 parts per million.
*St. Patricks well, Louisville9— (19000—16,400 parts per million.
*Carroll County artesian well10—16,000 parts per million.
Old Crow Distillery, Franklin County:11
a. (7900—1596 parts per million,
b. (7900—1610 parts per million.* Recalculated from grams per litre to parts per million.
As indicated elsewhere these deep tests may not all be St. Peter wells. In some places relatively fresh water is encountered in a sandstone in the Cotter (Knox). Similarly, water from the Lower Blue Lick Springs, Nicholas County, Kentucky, which has been interpreted as of deepseated (St. Peter-Calciferous) origin shows (Matson, 1909, quoting analyses of the Kentucky Agricultural Experiment Station): 10,296, 10,558, 9,026, 9,315 and 9,022 parts per million.
Analyses12 of brines from the old Glen Font salt works in Meade County and the Goose Creek salt works near Manchester, Clay County, show 81,900 and 84,400 parts per million respectively. The source of the former is probably "Corniferous" and the latter Pennsylvanian, probably one of the Salt Sands.
Several analyses11 of salt water from wells in the Irvine-Big Sinking and other pools of Lee, Estill, and Powell counties show:
*Well on H. Angel lease, Lee County (5000—87,800 parts per million.
*Watson No. 7 well, Estill County (±8500— 30,700 parts per million.
*Hall No. 10 well near Furnace, Estill County (±850')—55,100 parts per million.
* Recalculated from grams per litre to parts per million.
The source in the Irvine field is the Peebles (Guelph) and Lilley (Lockport) limestones, occurring here directly beneath the Ohio shale. In parts of the Big Sinking field the Mid-Devonian is also possibly present. The .precise levels from which the brines came are not known, nor is the quantity available known. The salinity of water from the Angel and Hall wells is reported to be of sufficient concentration to have possible commercial value. The concentration in the Angel well, which was the highest of the three is, however, but little more than one-third the average salinity shown by 38 analyses listed by Stout, et al. (1932) from the Second or Big water of the Big Lime (Newburg sand) in Ohio. This horizon is essentially that of the upper Peebles, in which lenticular sandstone is also found (p. 314). With increasing depth in the Eastern Goal Field greater salinities may be expected. The Niagaran, though, wedges out somewhere to the east and the horizon is represented by unconformity where it reappears in the western edge of the Appalachian folds. The iodine and bromine content from these samples and two others from northeastern Estill County showed (McHargue, Kentucky Agr. Exp. Sta.):
| Parts per million | ||
| Iodine | Bromine | |
| H. Angel well, Lee County | 1.3 | 9.7 |
| Watson No. 7 well, Estill County | 0.42 | 55.00 |
| Hall No. 10 well near Furnace, Estill County | 3.75 | 228.00 |
| John W. Rogers No. 6, Estill County | 2.00 | 50.00 |
| Frank Rogers No. 2, Estill County | 4.00 | 90.00 |
* Recalculated from grams per litre to parts per million.
The Pocono (Waverly—Big Injun, etc.) is not much of a brine producer in eastern
Kentucky, but, as noted above, is the important commercial brine at Pomeroy,
Meigs County, Ohio. Analyses listed by Stout et al. (1932) from that vicinity
gave 95,280 and 97,859 parts per million solid matter. Similarly, analyses from
counties in West Virginia which adjoin Kentucky show that the brine from Wayne
County has 107,881 and 59,584 parts per million solid matter; Lincoln, 12,386;
and Cabell, 103,789, 52,059, 157,304, 178,497, and 169,641. No analyses from
wells in Kentucky are available
The Berea locally yields brine in eastern Kentucky, but no analyses are available nor were many listed by Price et al. (1937a) from West Virginia. Stout et al. (1932) gave two analyses from counties in Ohio near the Kentucky line—Scioto County—102.0, and Meigs County—151.0, parts per million solid matter.
Brines are encountered locally in the Maxon (Maxton) in eastern Kentucky, but again the only comment possible is that concerning production and analyses in West Virginia. This sand (Pennington—Mauch Chunk) is apparently not developed in Ohio, though the term is used for one of the Salt Sands. No analyses are listed for counties in West Virginia immediately adjoining Kentucky. One in Cabell County (1195 to 1232 feet) has a salinity of 59,400, one in Boone County (1955 to 1965 feet), 167,000, and one in Kanawha County (1380 to 1390 feet), 57,600 parts per million. The gas- and oil-producing area of the Maxon, which is largely a matter of the availability of the sand, is shown on plate LXXXII.
The Salt Sands constitute the great salt water horizon of oil and gas exploration in eastern Kentucky. As indicated earlier, the brines are commercially important in both Ohio and West Virginia. Several analyses of brines from the Lower Salt Sand of Ohio gave an average of 63,210 parts per million and a maximum of 102,000. In West Virginia a large number are listed, some not far from the Kentucky line. In Cabell County six analyses ranging up to 86,900 parts per million average about 50,500.
Two in Logan County gave 159,600 and 137,100 respectively. A large number in Boone County average about 71,406 and have a maximum of 150,900. These included several that were quite dilute.
How Kentucky brines from these Salt Sands compare with those of Ohio and West Virginia is not known. Stout et al. (1932) found a relationship in Ohio between salinity and depth. This does not seem to be indicated in the West Virginia analyses, and it may be more a matter of distance from outcrop than actual depth. As such, there would exist the expectation of greater concentration with increase in depth and distance from outcrop in the interior of the Pittsburgh Basin and greater dilution marginally. If depth itself is significant, several things should be noted with regard to this basin, which is known under the name of Eastern Kentucky geosyncline in Kentucky.
(1) The regional eastward dip of the Ohio shale is unaffected by the reversal of the Pennsylvanian in the Eastern Kentucky geosyncline. Thus although surface outcrop shows an eastern rim of the basin, there is progressive increase in depth of deep pays eastward beneath this eastern rim. This would be significant for many of the deeper brine-producing beds.
(2) The Pittsburgh Basin was not a progressively developed one but formed at the time of the Appalachian Revolution in a region of minimum thickness of Pennsylvanian, thus previously a "high" (McFarlan, 1939). With progressive subsidence of the Appalachian geosyncline in the Pennsylvanian as shown in the rapidly increasing thickness of the Pottsville to the south and southeast the productive Salt Sands will lie deeper in some areas marginal to the basin as determined by the surface outcrop than in the center of the basin. Here the marginal-central relationship loses significance.
(3) The plane of the Pine Mountain thrust is a means of access and dilution by meteoric waters.
IRON ORE
As a mineral resource iron is only of historic interest—this in spite of the fact that Kentucky was a pioneer state in its production. The old Slate Creek furnace erected near 0wingsville in 1790 or 1791 was the first west of the Alleghenies. The ore bodies are small and scattered, and the large scale development of ores in the Lake Superior country eliminated the industry in Kentucky.13
 |
| FIG. 42 Old iron furnaces in Kentucky |
Preston Ore Banks (Boyle limestone).—The Preston ore banks were the first worked in Kentucky and were located on Slate Creek southeast of the present site of Owingsville. The ruin of the Slate Creek furnace still stands, with a good sized tree growing out of the top, on the bank of Slate Creek along the road between Preston Station and Owingsville. The ore was an oolitic carbonate, occupying the position of the Boyle limestone on the ridge to the east of the creek. Miller regarded it as a replacement of this limestone. The weathered ore in the form of limonite was first mined. Of historic interest is the shipment of cannon balls by way of the Licking and Ohio rivers to General Jackson for the defense of New Orleans. The deposit was worked intermittently and exhausted about 1894.
Rose Run Mines.—With exhaustion of the Preston ore banks operations were transferred to Rose Run about five miles northeast of Olympia on the Chesapeake & Ohio Railroad. The ore is the usual "Clinton" type, oolitic hematite ("flaxseed ore," "fossil ore," etc.), and the average thickness is three feet. This is the maximum and only commercial thickness known in Kentucky. It is in the Brassfield formation and thus stratigraphically below the horizon of the Big Seam of Birmingham, Alabama. Minor seams occur below the Big Seam in the Medina at Birmingham. The better ore runs 46 to 57 per cent ferric oxide and, because of its calcareous nature, was at one time shipped to Ashland to be smelted with Lake Superior ores. Mining was continued until about 1916 or 1917.
Nodular (Concretionary) Ores of the Lower Waverly.—Iron ore occurs in the lower Waverly as siderite concretions, which weather out to limonite. It was mined and smelted for a time at the Caney furnace southeast of Salt Lick, Bath County. Similar ores were worked for a time in Bullitt and Nelson counties.
Limonite Ores Occupying Erosion Hollows in the Mississippian-Pottsville Contact.—Miller (1919) regarded this ore as probably the carbonate formed by replacement of the limestone and converted into limonite by weathering. The ore was fairly abundant along the western margin of the Eastern Coal Field north of the Kentucky River and especially along the divide between that river and the Red River. This concentration north of the Kentucky River is probably related to the overlap northward of the Pottsville onto successively older beds. Southward an increasing thickness of Pennington shale intervenes between the limestone and Pottsville. Among furnaces built and operated in this region were:
(a) One at the present site of Clay City, 1808.
(b) Estill furnace, 1830 or 1831, on the top of the divide at what is now Furnace P. O., replacing the earlier one at Clay City.
(c) Fitchburg, 1869, on Furnace Fork of Millers Creek. It was short-lived.
Charcoal was used, and according to Miller (1919) the clearing of about eight-tenths of an acre (175 bushels of charcoal) was required for every ton of iron produced.
Farther north there was the Beaver furnace (1819) in northeastern Menifee County and the Bath furnace south of Salt Lick in Bath County. Other furnaces in Boyd, Carter, and Greenup counties used ores from a higher level in addition to those at this contact.
Ores of Boyd, Carter, and Greenup Counties.—The region where these ores were worked is the Kentucky extension of the Hanging Rock region of southeastern Ohio. Moore (1876) reported 13 furnaces in operation in that part of Kentucky, 11 using charcoal, the other two coal. These and earlier ones which had ceased operating at that time totaled seven in Boyd, 15 in Greenup, and three in Carter County. Several ores were used:
(1) Ore at the Mississippian-Pennsylvanian contact outlined above. The ore was essentially, restricted in occurrence to the region west of Tygarts Creek. In most of the northeastern area of outcrop the Pottsville rests on Waverly.
(2) Block ores. These are beds of carbonate ore three to 15 inches thick, weathering to limonite, called block ores because of their cubical cleavage. They occur at several horizons:
(c) Between the Lower and Upper Mercer coals
(b) Between the Quakertown and Lower Mercer coals.
(a) Between the Sharon and Quakertown coals.
(3) Ferriferous limestone ore—a bed of carbonate ore weathering to limonite. The ore ranges up to 5 feet thick and occurs at the horizon of the Vanport limestone of Pennsylvania and Ohio between the Brookville and Lower Kittanning coals. Operations were in eastern Greenup and Carter counties east of the Little Sandy River.
(4) Kidney ores. These are carbonate concretions occurring in shales and weathering to limonite. The Lower or Yellow Kidney ore lies between the Lower and Middle Kittanning coals and the Upper or Red Kidney ore between the Middle Kittanning and Lower Freeport coals. They occur mainly in Boyd and extreme southeastern Carter counties.
The industry in this region reached its maximum about 1884 and died out a few years later.
Lower Cumberland and Tennessee River Ores.—The development of this district mainly in "Between the Rivers" dates back to 1830. The ore was a limonite occupying the contact between the Mississippian limestone (here Keokuk) and the Cretaceous and Tertiary. The extensive cutting of timber for charcoal practically stripped the region, which is now reforested with a second growth. It is reported that in one of these furnaces William Kelley in 1851 discovered the "Bessemer" process of steel making, later independently discovered and accredited to Bessemer.
Muhlenberg County ore.—This is a siderite ore known as "black band" and forms the roof of No. 12 coal.
Footnotes
1 For a general discussion of building stone see C. H. Richardson (1923).
2 Some of the upper Ste. Genevieve probably included.
3 The Oregon limestone is being mined by shaft at Lexington.
4 Note According to A. F. Rogers (1917) the principal mineral is cellophane
(3Ca3(PO4)2-nCa(CO3,F2,SO4,O)•(H2O)x) accompanied in some instances by dahllite
(3Ca3(PO4)2•Ca(F2,O,CO3) •x H2O). Investigation by a number of workers in the
United States Bureau of Soils indicates the phosphate mineral is a fluor-phosphate
of the approximate composition Ca10F2(PO4)6 referred to as "sub-microcrystalline
fluorapatite."
Bibliographic references include:
Jacob, K. D., and Reynolds, D. S., The fluorine content of phosphate rock: Jour.
Assoc. Official Agr. Chem., vol. 11, pp. 237-250, 1928.
Reynolds, D. S., Jacob, K. D., and Hill, W. L., Ratio of fluorine to phosphoric
acid in phosphate rock: Ind. and Eng. Chem., vol. 21, p. 1253, 1929.
Hendricks, S. B., Hill, W. L., Jacob, K. D., and Jefferson, M. E., Structural
characteristics of apatite-like substances and composition of phosphate rock
and bone as determined from microscopical and X-ray diffraction examinations:
Ind. and Eng. Chem., vol. 23, p. 1413, 1931.
Marshall, H. L., Jacob, K. D., and Reynolds, D. S., Occurrence of fluorine in
natural phosphates; Further studies: Ind. and Eng. Chem., vol. 24, p. 86, 1932.
Hill, W. L., Marshall, H. L., and Jacob, K. D., Minor metallic constituents of
phosphate rock: Ind. and Eng. Chem., vol. 24, p. 1306, 1932.
Hill, W. L., and Jacob, K. D., Determination and occurrence of iodine in
phosphate rock: Jour. Assoc. Official Agr. Chem., vol. 16, pp. 128-137, 1933.
Hendricks, S. B., Hill, W. L., Jacob, K. D., and Jefferson, M. E., Idem, p.
1414, 1931.
5 The author is indebted to A. M. Peter for assistance with these notes.
6 Kentucky Agr. Exp. Sta., report 28, vol. 28, 1915, p. 50.
7 G. C. Matson, Water resources of the Blue Grass Region, with a chapter on the
quality of the waters by Chase Palmer, U. S. Geol. Surv., Water Supply Paper
233, 1909, pp. 212-213.
8 Charles Butts, Geology and mineral resources of Jefferson County, Kentucky,
Kentucky Geol. Surv., ser. 4, vol. 3, pt. 2, 1915, p. 245.
9 Kentucky Agr. Exp Sta., report 25, vol. 24-25, bul. 168, 1912, pp. 537-538.
10 Records of the Kentucky Agr. Exp. Sta.
11 Footnote 7, p. 430.
12 Analyses by Kentucky Agricultural Experiment Station.
13 A brief outline of the development of this industry in Kentucky is given by
A. M. Miller (1919), and the following notes are taken mainly from this work.