Chapter 27
Approach to the Patient with Hypercholesterolemia
Mason W. Freeman
Over the last several years, evidence has accumulated
demonstrating that treatment of hypercholesterolemia can reduce
atherosclerosis and its attendant cardiovascular complications (see Chapter 15).
These findings have heightened physician and patient awareness of the
importance of hypercholesterolemia. The primary care physician needs to
be capable of evaluating hypercholesterolemia and of designing and
implementing a treatment program that effectively uses dietary
treatment, exercise, weight loss, and, when necessary,
cholesterol-lowering drugs.
PATHOPHYSIOLOGY (1,2,3,4,5,6,7,8)
The production of atherogenic lipoproteins and the
induction of atheromatous plaques by those lipoproteins involve
distinct pathways. The presence of an elevated serum cholesterol level
does not, by itself, guarantee the development of atherosclerotic
lesions that will become clinically important any more than a normal
cholesterol concentration ensures plaque-free coronary arteries. The
formation and subsequent rupture of atherosclerotic lesions, leading to
the acute coronary syndromes of
unstable angina and myocardial infarction, depend on complex cellular and metabolic interactions. Serum lipids, inflammatory cells recruited to the sites of lipid deposition, the normal cellular constituents of the artery wall, and components of the blood coagulation system all contribute to the pathogenesis of atherosclerosis and its clinical consequences.
P.191
unstable angina and myocardial infarction, depend on complex cellular and metabolic interactions. Serum lipids, inflammatory cells recruited to the sites of lipid deposition, the normal cellular constituents of the artery wall, and components of the blood coagulation system all contribute to the pathogenesis of atherosclerosis and its clinical consequences.
Lipoproteins
An understanding of lipoproteins and their metabolism
helps to guide physicians in evaluating and treating lipid disorders.
To circulate in the aqueous environment of the blood, nonpolar lipids
such as cholesterol and triglyceride are complexed with proteins and
the more polar phospholipids into spheres called lipoproteins. The protein components of the lipoproteins are known as apoproteins,
which play both structural and functional roles in the metabolism of
lipid particles. Genetically inherited mutations in either the
structure of apoproteins or the receptors that bind them account for
many of the most severe forms of hyperlipidemia. The lipoproteins are
usually divided into four major classes based on particle density,
which is a reflection of their relative protein and lipid content: chylomicrons, very low density lipoproteins (VLDLs), low-density lipoproteins (LDLs), and high-density lipoproteins (HDLs). There are also subdivisions and minor classes of lipoproteins (Table 27.1).
Table 27.1. Lipoprotein Composition | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Chylomicrons
Chylomicrons derive from dietary fat and carry
triglycerides throughout the body. They have the lowest density of all
lipoproteins and will float to the top of a plasma specimen left in the
refrigerator overnight. The chylomicron itself is probably not
atherogenic, but the role of the triglyceride-depleted chylomicron
remnant is uncertain. Triglyceride makes up most of the chylomicron and
is removed by the action of lipoprotein lipase. Patients deficient in
this enzyme or its cofactors (insulin and apolipoprotein CII) have very
high serum triglyceride levels and increased risk of acute pancreatitis.
Very-Low-Density Liproproteins
VLDLs are also triglyceride rich and are acted on by
lipoprotein lipase. Their function is to carry triglycerides
synthesized in the liver and intestines to capillary beds in adipose
tissue and muscle, where they are hydrolyzed. After removal of their
triglyceride, VLDL remnants can be further metabolized to LDL. The
atherogenicity of native VLDL is controversial, but the metabolism of
VLDL to atherogenic lipoproteins is not in doubt. VLDLs serve as
acceptors of cholesterol transferred from HDL, possibly accounting in
part for the inverse relation between HDL cholesterol and VLDL
triglyceride. The serum enzyme cholesterol ester transfer protein
mediates the process.
Low-Density Lipoproteins
LDLs are the major carriers of cholesterol in humans.
They carry cholesterol to tissues and deliver it via receptors on the
cell surface that bind and internalize the LDL particle. LDLs are the
lipoproteins most clearly implicated in atherogenesis. LDL levels are
increased in individuals who consume large amounts of saturated fat
and/or cholesterol. There are also several Mendelian genetic disorders
that result in increased LDL levels. These disorders include mutations
that produce defective LDL receptors (familial hypercholesterolemia) or
mutant proteins that interact with the LDL receptor (autosomomal
recessive hypercholesterolemia). LDL levels can also result from
genetically encoded abnormalities in the structure of LDL apoprotein B.
Finally, there are non-Mendelian, polygenic disorders that cause
increases in LDL.
When serum LDLs exceed a threshold concentration, they
traverse the endothelial wall and can become trapped in the arterial
intima. There, they may undergo oxidation, aggregation, or other
modifications that cause their uptake by macrophages. This process
appears to be an important initiating step in atherogenesis. The
association of serum total cholesterol with coronary heart disease
(CHD) is predominantly a reflection of the role of LDL because LDL
cholesterol constitutes the bulk of serum cholesterol in most humans.
Many well-designed studies demonstrate that lowering the LDL
cholesterol can dramatically reduce subsequent coronary events and
all-cause mortality in hypercholesterolemic patients.
High-Density Lipoproteins
HDLs appear to function in peripheral tissues as an
acceptor of free cholesterol that has been transported out of the
cellular membrane. The cholesterol is esterified and stored in the
central core of the HDL and may be further metabolized. This reverse
transport system may explain why patients with very high HDL levels
have a reduced risk of developing CHD, even if their LDL levels are
elevated.Apolipoprotein AI is the major
apoprotein of HDL, and its level also inversely correlates with the
risk of CHD. Women have higher levels of HDL cholesterol than men, in
part because of their higher estrogen levels. Exercise increases HDL,
whereas obesity, hypertriglyceridemia, and smoking lower HDL. The
HDL-cholesterol concentration is the single most powerful lipid
predictor of CHD risk, but therapies that raise HDL-cholesterol levels
have proven difficult to develop, and the significance of such an
intervention on coronary disease outcomes is uncertain.
P.192
Dietary Influences
Dietary fat and cholesterol have a substantial influence
on serum- and LDL-cholesterol levels. Saturated fat intake has a
greater effect on serum cholesterol than does dietary cholesterol
intake. For each increase in percentage of total calories contributed
by saturated fats, serum cholesterol increases by a factor of 2.16,
whereas the serum cholesterol increase is only 0.068 for each
percentage increase in dietary cholesterol. This relationship is
summarized in the equation of Hegsted:
Change in total cholesterol = 2.16 delta S – 1.65 delta P + 0.068 delta C
where delta S, delta P, and delta C are the changes in
the percentage of total calories contributed by saturated fats,
polyunsaturated fats, and cholesterol, respectively. Fats are
characterized by their constituent fatty acid composition. The fatty
acids are characterized as saturated, polyunsaturated, or
monounsaturated. The state of saturation refers to the number of
carbon–carbon double bonds contained in the fatty acid.
Saturated Fatty Acids
Saturated fatty acids can raise LDL cholesterol, in part
by altering the LDL receptor's catabolic activity. The long-chain
saturated fatty acids common to the American diet—lauric (12 carbons),
myristic (14 carbons), palmitic (16 carbons), and stearic (18
carbons)—have no double bonds and are not essential for human growth
and development. Not all saturated fatty acids trigger rises in LDL
cholesterol. For example, stearic acid and some shorter chain fatty
acids (caproic and caprylic) do not. In the typical American diet,
about one-third of the saturated fat content derives from meat and meat products, whereas another one-third comes from dairy products and eggs, and 10% from baked goods. Vegetable oils also may contain saturated fat (see Appendix I), especially the so-called “tropical oils”
(coconut and palm) and cocoa butter, which are commonly used in
commercial food preparation. Even when unsaturated oils (see later
discussion) are used in processed foods, they usually undergo partial hydrogenation,
which adds back hydrogens to the carbon–carbon double bonds,
eliminating some double bonds and making the fatty acids more
saturated. This saturation process is performed to make these oils more
solid at room temperature, but it also makes them more
hypercholesterolemic.
Monounsaturated Fatty Acids
Monounsaturated fatty acids are present in all animal and vegetable fats. The most common dietary form is oleic acid,
plentiful in peanuts, almonds, olives, and avocados. Oils derived from
these sources neither raise nor lower LDL cholesterol by themselves,
although cholesterol and CHD risk fall if they are used as substitutes
for saturated fat. Mediterranean diets rich in olive oil and other sources of monounsaturated fatty acids appear to be relatively nonatherogenic, even though they are not low in fat.
Polyunsaturated Fatty Acids
Unlike saturated and monounsaturated fatty acids,
polyunsaturated fatty acids (PUFAs) are not synthesized by the body.
They must be present in the diet and are referred to as essential fatty acids.
The location of the first double bond from the methyl end of the
molecule determines the nomenclature of the PUFAs. The major dietary
fatty acids contain either an n-6 or n-3 first double bond. Linoleic and arachidonic acids are the common Ω-6 PUFAs, found in considerable quantities in liquid vegetable oils
(sunflower, safflower, corn, and soybean). The Ω-3 fatty acids are
represented by linoleic acid (found in canola oil and leafy vegetables)
and the Ω-3 fish oils (eicosapentanoic and
docosahexanoic acids). The latter attracted considerable interest when
epidemiologic studies found a link between their consumption and
reduced rates of CHD mortality.
When vegetable oils rich in PUFAs are subjected to partial hydrogenation in commercial food processing, not only do some of their double bonds get converted to single bonds, but others shift from the cis configuration to the trans
configuration, which increases their atherogenicity and associated CHD
risk. Intake of such substances increases LDL cholesterol,
lipoprotein(a) (LPa), and triglycerides and reduces HDL cholesterol.
Data from the Nurses' Health Study suggest that replacing trans
unsaturated fats in the diet with polyunsaturated fats can reduce CHD
risk by nearly 60%, a much greater reduction than even that achieved by
reducing overall fat intake.
Cholesterol
As the Hegsted formula indicates, dietary cholesterol
has a much smaller effect than saturated fatty acids on raising total
cholesterol. For every additional 100 mg of dietary cholesterol
consumed per day, the serum cholesterol will rise by about 8 to 10
mg/dL. However, organ meats (e.g., brain, kidney, heart, sweetbreads) and egg yolks are concentrated sources of dietary cholesterol (see Appendix II) and can have a substantial effect on serum cholesterol levels. Although shellfish
contain moderate amounts of cholesterol, they have relatively small
amounts of saturated fat and are sources of Ω-3 PUFAs. Cholesterol is
absent from food derived from plants. Plant stanols and sterols can
actually block cholesterol absorption in the intestine, and a
commercially available margarine containing the plant stanol sitostanol
is available as a cholesterol-lowering agent. It reduces serum
cholesterol levels by 10% to 15%. Recently released National
Cholesterol Education Program (NCEP) guidelines (Adult Treatment Panel
[ATP] III) encourage the use of these plant stanols in dietary programs
aimed at reducing blood cholesterol levels.
Other Dietary Factors
Low-fat, high-carbohydrate diets
can reduce HDL cholesterol and increase triglycerides. Especially in
obese persons, increased total caloric intake may induce overproduction
of VLDL triglycerides while reducing HDL cholesterol levels. Data from
the Nurses' Health Study suggest that substituting carbohydrate for saturated fat in the diet may reduce CHD risk by about 15%, but substituting carbohydrate for polyunsaturated fat
may increase CHD risk by greater than 50%. There is no evidence that
either dietary carbohydrate (whether simple sugars or complex ones) or
protein significantly affects LDL cholesterol.
The fiber content of food has generated much interest. Insoluble fiber
(typically cellulose found in wheat bran) has no cholesterol-lowering
effect, although it is beneficial for lowering the risk of diverticular
disease and colon cancer (see Chapter 65). Soluble fiber (pectins, certain gums, psyllium) has received much attention in the lay press stimulated by claims about oat bran,
which contains the gum beta-glycan. Initial studies were encouraging,
but subsequent data suggested the cholesterol decreases observed were
no greater than those found with use of insoluble fiber and probably
resulted from replacement of dietary fat in the diet rather than from a
direct effect on lipid metabolism. When studied in patients already
taking a low-fat
diet, high-soluble-fiber intake appeared to lower serum cholesterol by a modest amount (3% to 7%).
P.193
diet, high-soluble-fiber intake appeared to lower serum cholesterol by a modest amount (3% to 7%).
WORKUP (2,9,10,11,12,13,14)
Diagnosis
The diagnosis of hypercholesterolemia (see also Chapter 15) should always be based on repeat measurements
of serum lipids because combined analytic and biologic variations in
serum lipids range from 10% to 20%. A single measurement should never
be viewed as sufficient for a diagnosis of hypercholesterolemia. A venous sample processed in a laboratory meeting Centers for Disease Control and Prevention standards for cholesterol determination (see Chapter 15) is recommended.
Although earlier guidelines often recommended a stepped
approach to performing lipid analyses in patients, the ATP III
guidelines now suggest that a fasting lipid profile be done at the
initial assessment, whenever possible (Table 27.2). A fasting venous sample for determination of serum cholesterol, HDL cholesterol, and triglycerides
(with calculation of LDL cholesterol derived from these determinations;
see later discussion) constitutes the traditional lipid profile and the
basis for estimating VLDL cholesterol and calculation of LDL
cholesterol. The results characterize the lipid disorder and guide
treatment decisions. If a fasting lipid profile cannot be readily
arranged, then a practical alternative in persons at low CHD risk is to
obtain nonfasting determinations of total cholesterol and HDL
cholesterol and reserve for a full lipid profile only those persons
with a nonfasting total cholesterol greater than 200 mg/dL or an HDL
cholesterol less than 40 mg/dL (see Chapter 15).
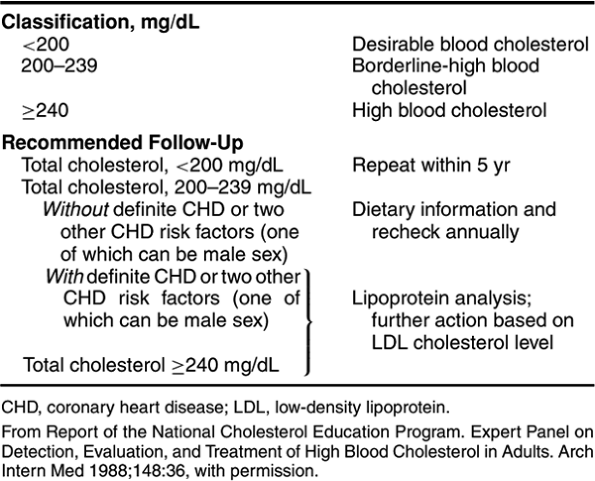 |
Table 27.2. Initial Classification and Recommended Follow-Up Based on Total Cholesterol |
Estimation of VLDL cholesterol and calculation of LDL cholesterol
are derived from measurements of total- and HDL-cholesterol levels and
the triglyceride concentration using the following formula:
LDL cholesterol = total cholesterol – (HDL cholesterol + triglyceride/5)
The triglyceride/5 factor represents a close estimate of
VLDL cholesterol and derives from the observation that VLDL cholesterol
is usually 20% of the serum triglyceride value. The validity of this
formula for estimating LDL cholesterol has been confirmed by direct
LDL-cholesterol measurement and remains fairly accurate so long as the
total triglyceride is less than 400 mg/dL. A fasting
sample is required for accurate results because chylomicrons appearing
in the blood after a meal do not contain the same ratio of triglyceride
to cholesterol found in VLDL. If the triglyceride level is greater than
400 mg/dL, the LDL cholesterol can be determined accurately only by
more sophisticated and expensive methods. These methods include
immunologic and nuclear magnetic resonance quantitation of LDL, gel
filtration techniques, and ultracentrifugation. The added cost of these
measures generally mitigates against their routine use, but they can be
useful in patients with elevated triglycerides or unusual clinical
presentations.
Excluding Secondary Causes
Before embarking on a treatment plan, one must exclude
conditions that might secondarily lead to hyperlipidemia. The most
important are hypothyroidism, nephrotic syndrome, and diabetes (Table 27.3), best screened for by a serum thyroid-stimulating hormone, urine dipstick for protein, and serum glucose, respectively (see Chapters 93, 104, and 130). Drugs can affect lipid levels as well, with LDL elevations occurring with thiazide use and triglyceride levels rising with beta-blockers. Postmenopausal estrogen replacement
lowers LDL and increases HDL and triglycerides. Antiviral protease
inhibitors used in the treatment of AIDS often cause hyperlipidemia as
well.
Table 27.3. Classification of Lipoprotein Disorders | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Classification
The original Fredrickson classification
scheme is of limited utility now that a better understanding of the
genetics of these diseases has emerged. However, no unified
classification of comparable simplicity has replaced it. For most
clinical purposes, it is probably simplest to separate patients into
three broad categories: those with elevated cholesterol, elevated cholesterol and triglyceride, and elevated triglyceride only. Table 27.3
summarizes the likely diagnoses under these broad categories. The
possibility of a genetic disorder should be considered if extremes of
any lipid level are encountered or if there is a history of premature
CHD in the patient or the family.
Risk Stratification
Risk stratification can be of considerable help to both
patient and physician, helping to provide an evidence-based rationale
for choice and intensity of treatment (see Tables 27.4 and 27.5).
Benefit from treatment of hypercholesterolemia is closely linked to the
degree of pretreatment CHD risk, making a careful assessment of that
risk essential.
Table 27.4. Coronary Heart Disease Risk Associated with Lipoprotein Cholesterol Abnormalities | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
Table 27.5. Coronary Heart Disease Risk Factors and Coronary Heart Disease Risk Status | |||
|---|---|---|---|
|
Role of Established CHD Risk Factors in Risk Stratification
The CHD risk assessment should be a comprehensive one,
including but also extending beyond lipid levels to consideration of
all major established risk factors, including hypertension,
smoking, diabetes, family history of premature CHD, age, gender, and
presence of established CHD or other atherosclerotic disease (e.g., peripheral arterial insufficiency, symptomatic carotid disease; see Appendix of Chapter 26 for calculation of risk). The increasing awareness of elevated HDL cholesterol as a factor in reducing CHD risk has led to its designation as a “negative risk factor”; conversely, a low HDL cholesterol
enters the list of positive risk factors (Table 27.5 and Appendix of Chapter 26).
P.194
enters the list of positive risk factors (Table 27.5 and Appendix of Chapter 26).
A gradient of CHD risk has been defined by the NCEP
expert panel, taking into account degree of LDL elevation and presence
of other CHD risk factors (Tables 27.4 and 27.5 and Appendix of Chapter 26). For a given elevation in LDL cholesterol level, patients can be additionally classified according to total CHD risk:
- Very high risk: acute coronary insufficiency or CHD plus multiple severe risk factors.
- High risk: established CHD or CHD risk equivalents (e.g., diabetes mellitus, peripheral or carotid atherosclerotic disease, multiple CHD risk factors with a 10-year CHD risk of >20%).
- Moderately high risk: no established CHD, but two or more CHD risk factors giving a 10-year CHD risk of 10% to 20%).
- Moderate risk: no CHD, but two or more CHD risk factors and 10-year risk less than 10%.
- Lowest risk: no CHD risk factors
Risk stratification by use of the established risk
factors does not fully account for all observed CHD risk. Efforts to
identify other independent risk factors are ongoing, with C-reactive
protein and homocysteine receiving the most attention.
Role of C-Reactive Protein in Risk Stratification
Epidemiologic evidence supports C-reactive protein (CRP) as an
independent risk factor for CHD events, particularly in women. However, the association between CHD risk and CRP elevation appears less pronounced than originally proposed (relative risk on the order of 1.5 vs previous estimates of 2 to 2.5) and weaker than that for the more established CHD risk factors. Moreover, there are no definitive data yet from long-term randomized clinical trials proving that lowering of CRP by itself reduces risk of CHD events, nor is it clear how to reduce CRP. Nonetheless, CRP determination may be helpful in selective instances where the results will change management of established treatable CHD risk factors (e.g., motivate the patient to make substantive lifestyle changes or trigger more aggressive pharmacologic treatment of modifiable CHD risk factors). If measured, the high-sensitivity assay for CRP should be used.
P.195
independent risk factor for CHD events, particularly in women. However, the association between CHD risk and CRP elevation appears less pronounced than originally proposed (relative risk on the order of 1.5 vs previous estimates of 2 to 2.5) and weaker than that for the more established CHD risk factors. Moreover, there are no definitive data yet from long-term randomized clinical trials proving that lowering of CRP by itself reduces risk of CHD events, nor is it clear how to reduce CRP. Nonetheless, CRP determination may be helpful in selective instances where the results will change management of established treatable CHD risk factors (e.g., motivate the patient to make substantive lifestyle changes or trigger more aggressive pharmacologic treatment of modifiable CHD risk factors). If measured, the high-sensitivity assay for CRP should be used.
Role of Homocysteine in Risk Stratification
Elevations in homocysteine are associated with statistically significant but modest increases in risk of CHD events (see Chapter 15).
Folic acid supplementation is capable of reducing elevations in
homocysteine, but there is no evidence yet from prospective, randomized
trials that reduction in homocysteine elevation reduces CHD risk.
Routine measurement of homocysteine levels is not warranted at this
time, but selective testing may be worth consideration when there is a
strong family history of CHD, onset of CHD is premature, or in the case
of a patient with CHD but no identifiable risk factors.
PRINCIPLES OF MANAGEMENT
Overall Approach (2,7,8,9,15,16,17)
The goals are to reduce coronary morbidity and mortality. Both primary prevention (reducing risk of having a first coronary event) and secondary prevention
(reducing risk of a new coronary event in a person with established
CHD) are sought. The most impressive reductions in risk are achieved in
patients at greatest risk (see later discussion), and the greatest
reductions are being increasingly realized with intensive lipid-lowering therapy in such persons.
The growing appreciation of the importance of lipid
abnormalities and the benefits of treating them have stimulated the
National Institutes of Health to sponsor the third formulation of the
ATP guidelines of the NCEP. Many of the treatment recommendations
generated by that expert panel are included here.
As noted earlier, the approach to treatment of hyperlipidemia is guided by an assessment of total CHD risk,
not just the lipid abnormality. For a given degree of LDL-cholesterol
elevation, the threshold for initiation of therapy decreases, and the
intensity of therapy increases with increasing CHD risk.
The NCEP treatment recommendations follow directly from
the degree of estimated CHD risk. Dietary modification is the sole mode
of therapy for patients at the lower end of the CHD risk spectrum,
whereas pharmacologic measures are reserved for patients at higher risk
or for those who fail dietary intervention (Table 27.6).
Additional considerations include possible adverse effects of long-term
pharmacologic therapy (an issue when dealing with young persons) and
appropriateness of the patient for treatment (an issue in the frail
elderly and seriously ill).
Table 27.6. Treatment Recommendations | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Dietary modification, complemented by exercise and
weight reduction, is the core of the lipid treatment program, with
pharmacologic therapy reserved for those at higher risk and for persons
failing behavioral therapies.
Dietary Modification, Exercise, And Weight Loss (4,6,9,18,19,20,21,22,23,24,25)
Dietary modification remains the cornerstone of
treatment, effective for both treatment and prevention of
hypercholesterolemia. As suggested by the Hegsted equation (see earlier
discussion), the greatest contributor to hypercholesterolemia is
the consumption of saturated fat, with excess cholesterol contributing to a lesser extent. Reductions in total fat, saturated fat, partially hydrogenated unsaturated fatty acids, and dietary cholesterol are recommended for all adults. Not only is it important to reduce total fat in the diet, but perhaps it is even more critical to substitute foods that provide polyunsaturated and monounsaturated fats for those rich in saturated and trans unsaturated fat.
P.196
the consumption of saturated fat, with excess cholesterol contributing to a lesser extent. Reductions in total fat, saturated fat, partially hydrogenated unsaturated fatty acids, and dietary cholesterol are recommended for all adults. Not only is it important to reduce total fat in the diet, but perhaps it is even more critical to substitute foods that provide polyunsaturated and monounsaturated fats for those rich in saturated and trans unsaturated fat.
In conjunction with exercise and weight loss
(which contribute to reductions in lipid levels and ameliorate other
cardiac risk factors), dietary modification provides an excellent
nonpharmacologic means of improving the patient's lipid profile and
reducing CHD risk. The adverse effects are nil, making it the safest of
treatments for hypercholesterolemia and especially well suited for
persons with only a modest increase in CHD risk (e.g.,
hypercholesterolemic young men and premenopausal women with no other
CHD risk factors). Even for high-risk patients, diet is central to the
treatment program, having an additive effect when combined with drug
therapy.
Efficacy
Decreases in intake of cholesterol and saturated fat in
controlled settings can reduce total and LDL cholesterol by 15% to 30%,
but the reductions average about 10% when similarly intensive dietary
programs are prescribed for outpatient use. Although reductions in
total and saturated fat are important, substituting polyunsaturates and
monounsaturates for saturated and unsaturated fats appears to be as or
even more important in reducing CHD risk by dietary intervention. Data
from the Nurses' Health Study provide very encouraging estimates of
expected changes in CHD risk from various dietary substitutions:
- Substituting polyunsaturated fat for saturated fat: 42% decrease in CHD risk (for every 5% of total calories)
- Substituting polyunsaturated fat for trans unsaturated fat: 57% decrease in CHD risk (for every 2% of total calories)
- Substituting carbohydrate for saturated fat: 17% decrease in CHD risk
- Substituting carbohydrate for polyunsaturated fat and monounsaturated fat: 20% to 60% increase in CHD risk
Note that not all substitutions are beneficial. Simply
substituting carbohydrate for all fat might not be the best dietary
strategy for reducing CHD because beneficial polyunsaturates would also
be eliminated.
The net effects of various dietary substitutions are
related in part to their effects on CHD risk factors, including HDL
cholesterol, LDL cholesterol, triglycerides, lipoproteins, and
platelets and clotting factors. For example, substituting total fat
intake with carbohydrate may produce a small (approximately 5%)
reduction in HDL cholesterol, although the overall total-to-HDL
cholesterol ratio typically still improves. Caloric and fat
restrictions are also effective in lowering triglyceride levels, an
effect enhanced by prohibition of alcohol. Reductions in CHD risk
parallel the degree of cholesterol lowering and reduction of other risk
factors.
Response to dietary modification is determined to some
extent by the etiology of the hypercholesterolemia. When the phase I
diet (see later discussion) is prescribed for outpatient use in
patients other than those with monogenic hypercholesterolemia, the
total and LDL-cholesterol levels fall by 5% to 15%. Typically, the
total serum cholesterol level will fall to 140 to 160 mg/dL in normal
individuals consuming a very low fat (5% to 10% of total calories)
diet. More modest but still useful reductions can be expected from less
stringent diets. In the setting of a metabolic ward study, there is
wide variability in the magnitude of LDL-cholesterol reductions
achieved by lowering saturated fat intake, ranging from less than 5% to
greater than 50%. Patients with severe monogenic hypercholesterolemias
rarely respond to diet alone, whereas other individuals consuming a
high-fat diet may demonstrate marked benefit.
Phased Approach to Dietary Modification
The phased approach, as exemplified by the American
Heart Association's three-phase dietary plan, maximizes adherence.
Total fat, saturated fat, and cholesterol intake are gradually reduced
with partial replacement by polyunsaturated fats (which by the Hegsted
equation have a modest cholesterol-lowering effect). Excess dietary
saturated and trans unsaturated fats are
supplanted by use of polyunsaturated and monounsaturated fats and by
complex carbohydrates (fruits, vegetables, cereals, pasta, grains, and
legumes).
Phase I Diet
Given the high prevalence of undesirable cholesterol
levels in the U.S. population, it is recommended that all Americans
adopt the phase I diet (Table 27.7):
Table 27.7. American Heart Association Three-Phase Dietary Plan | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
- Total fat as a percentage of total calories is reduced to 30% from an average 40% to 45%
- Saturated fat is reduced to 10% of total calories
- Polyunsaturated fat is increased to 10% of calories
- Dietary cholesterol intake is reduced from 500 to 300 mg/d
- Protein is held constant
- The Ω-6 PUFAs found in vegetable oils should not exceed 10% of calories because they may lower HDL cholesterol
The phase I diet usually does not require a dramatic
alteration in eating habits and can be readily adopted by most persons.
It is important to note that much of the polyunsaturated fat found in
“low-cholesterol, low-fat” processed food products is usually partially
hydrogenated, converting otherwise beneficial vegetable oils into the
undesirable trans unsaturated
configuration. Such processed food products should not be considered a
substitute for saturated fat, but neither should saturated fat be
considered a healthier alternative to such products and trigger
reverting back to lard-based or tropical oil–based processed foods.
Phase II Diet
The phase II diet (Table 27.7)
entails more effort because it goes beyond eliminating the obvious
sources of fat and cholesterol. It is indicated for patients who do not
achieve adequate results with a phase I diet and for patients at
highest CHD risk (e.g., established CHD).
If just the phase I dietary interventions were widely
implemented, the overall incidence of CHD in the population would
probably drop significantly. The recommended percentages of fat intake
in the diet must be translated into real menus and food recommendations
if good compliance with these recommendations is to be realized. The
counsel of a dietitian is often beneficial, particularly if a phase II
diet is indicated. A good working knowledge of the fat content of
common foods is essential for patient, family, and health care team
(see Appendix III). A number of
“heart-healthy” cookbooks are on the market to help patients in their
food choices and preparation. The use of faddish cholesterol cookbooks
should be discouraged because these often do not promote sustainable
healthy eating habits. Increasingly, restaurants are offering low-fat
menu choices, and patients should be encouraged to select them.
P.197
Low-Carbohydrate Diets
The observation that high-carbohydrate intake can
stimulate weight gain and raise LDL cholesterol and triglyceride levels
has led to interest in low-carbohydrate diets. Unfortunately, the most
popular low-carbohydrate diets (e.g., the Atkin's diet) substitute
liberal amounts of saturated fat for carbohydrates. Although short-term
(6- to 12-month) studies of obese persons using such diets demonstrate
weight loss, reduction in triglycerides, and improved glucose tolerance
without a significant increase in LDL cholesterol, there remain
concerns about the long-term durability and cardiovascular safety of
the low-carbohydrate/liberal saturated-fat approach. More studies are
needed, including long-term trials and examination of modest
carbohydrate restriction in conjunction with limitations on saturated
fat.
Nonprescription Dietary Supplements
Nonprescription dietary supplements are no substitute
for dietary reduction in total fat, saturated fat, and cholesterol.
Nonetheless, they are popular with patients, even though they can be
expensive.
Omega-3 Fish Oils
Preliminary data from prospective studies of omega-3
fish oil supplements are encouraging, but they are too limited to serve
as the basis for dietary recommendations. Impairment of clotting has
been noted with use of high doses.
“Antioxidant” Vitamins
Vitamins C, E, and beta-carotene
(the so-called “antioxidant vitamins”) do not lower cholesterol levels,
but they are capable of increasing LDL resistance to oxidative change
and theoretically might reduce the risk of arterial wall injury. Data
from small-scale human studies of vitamin E suggested a possible
reduction in CHD risk, but early prospective, large-scale, randomized
trials have so far failed to confirm a significant benefit; the same
pertains to vitamin C. The typical dose of vitamin E used in these
studies is 400 to 800 IU/d. There is no evidence that beta-carotene
reduces CHD events, and some data suggest an association with lung
neoplasms; its use for CHD prevention is not recommended. More data on
vitamin E and C will be forthcoming and should further clarify their
value, if any, in preventing coronary events.
Garlic, Fiber, and Yeast Supplements
One-half to one clove of garlic
a day may produce a modest (5%) reduction in serum cholesterol, but
powder and oil preparations have not demonstrated consistent
significant benefit. Use of fiber preparations such as psyllium (10 g/d) also provides modest benefit, as does the soluble fiber in oat-containing cereals. Red yeast extracts
contain a cholesterol synthesis inhibitor that is a member of the
statin family and can lower LDL 10% to 15%. Alcohols contained in sugar
cane (policanosols) can reduce LDL cholesterol by 20% to 30%, but data
are needed on their safety and long-term efficacy.
Exercise and Weight Loss
ATP III emphasizes exercise and weight reduction as
complements to dietary therapy and essential components of a
comprehensive nonpharmacologic program. They are helpful not only in
correcting lipid abnormalities, but also in reducing other CHD risk
factors and total CHD risk. For example, exercise will raise the level
of HDL cholesterol, decrease blood pressure, and increase efficiency of
peripheral oxygen extraction (see Chapter 18). Weight loss efforts can lower fat intake, reduce risk of diabetes mellitus, and decrease myocardial work.
Pharmacologic Therapy: Principles (2,9,15,16,17,20,26)
Dietary modification is not uniformly effective in
achieving target reductions in LDL cholesterol or desired increases in
HDL cholesterol. Addition of drug therapy to a diet and exercise
program should be considered in high-risk patients whose lipid
abnormalities remain inadequately controlled despite intensive dietary
efforts. Use of lipid-lowering therapy in patients at lower levels of
risk has become increasingly common due to the publication of
large-scale trials showing impressive reductions in CHD morbidity and
mortality in patients without established coronary disease (i.e.,
primary prevention studies) and modest levels of hypercholesterolemia.
Many lipid experts are treating more aggressively than is recommended
by the ATPIII guidelines, using outcomes of studies published after the
guidelines were generated to justify this approach.
Effectiveness and Safety
The addition of drug therapy to a diet and exercise
program can greatly enhance lipid-lowering results and lead to
significant reductions in nonfatal and fatal cardiac events (i.e., myocardial infarction, revascularization, cardiac death). Reductions in all-cause mortality
have also been demonstrated in lipid-lowering drug trials, particularly
in higher-risk populations. With intensive drug therapy, the rate of
plaque progression falls, and modest plaque regression
can be demonstrated in major coronary vessels and systemic arteries.
There is now evidence that lipid-lowering medication (statins) also can
reduce risk of stroke in persons with atherosclerotic carotid disease.
The reductions in CHD events have been noted in patients with established atherosclerotic disease (secondary prevention),
who experience 30% to 40% reductions in coronary morbidity and
mortality. In addition, benefit also accrues to persons with no
clinical CHD and only moderate increases in CHD risk, whose reductions
in rates of fatal and nonfatal coronary events with use of
pharmacologic therapy (primary prevention) are on the order of 20% to 30%.
P.198
An early concern about an increased risk of cancer and
violent deaths with use of some lipid-lowering agents has failed to
materialize in major prospective, randomized, placebo-controlled
studies. In four of the largest such trials conducted to date, using
several different members of the statin class of drugs and collectively
involving tens of thousands of patients (the Scandinavian Simvastatin
Survival Study and West of Scotland, AFCAPS/TexCAPS, and LIPID
studies), no increase in rates of cancer or noncardiac deaths has been
reproducibly observed. However, each class of drug therapies has
important adverse effects that need to be taken into account when
considering pharmacologic intervention (see later discussion). With the
trend toward more aggressive lowering of LDL cholesterol comes an
increase in risk of drug side effects as larger drug doses are
prescribed.
Candidacy for Pharmacologic Therapy
The risk-to-benefit ratio for pharmacologic therapy
appears most favorable in patients at greatest CHD risk and least
favorable in those at lowest risk. Because most data derive from
studies involving middle-aged men and postmenopausal women with established CHD or multiple CHD risk factors,
one must extrapolate to estimate effects in other populations.
Treatment recommendations by NCEP are based on total CHD risk that
includes lipid profile and associated cardiac risk factors.
In the elderly, high CHD
risk is common. One might reasonably expect the benefit noted in
high-risk, middle-aged patients to accrue also to elderly persons at
similar risk. These expectations have largely been borne out in the
studies that have included large numbers of older individuals (Heart
Protection Study). Duration of therapy is less likely to be very
prolonged, lessening risk of an adverse effect from long-term use.
Young men (age <35 years) and premenopausal women
with no CHD risk factors other than hypercholesterolemia are best
considered for nonpharmacologic therapy because their short-term risk
of CHD is quite low, and the safety of very-long-term drug therapy is
not established. Because the first statin was approved for clinical use
more than 20 years ago, the concern over potential adverse long-term
treatment effects is rapidly diminishing. For young persons at greater
CHD risk (e.g., LDL cholesterol >220 mg/dL, potent CHD risk factors
such as diabetes mellitus, or strong family history of premature CHD),
the potential gain from use of lipid-lowering medication almost
certainly outweighs the speculative long-term risks, but there are no
outcomes data yet available to confirm this. However, in women of
childbearing age, the potential teratogenicity of statin therapy must
always be kept in mind when prescribing that class of lipid-lowering
agent. The exercise of good clinical judgment is essential in these
circumstances.
Pharmacologic Therapy: Available Agents (2,9,18,20,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41)
Design of a pharmacologic regimen must take into account the patient's degree of CHD risk, the nature of the lipoprotein abnormality, and a drug's mechanisms of action and side effects.
The best program is one that addresses and fits well into the patient's
overall clinical state. A large degree of individualization is
necessary. The range of available drugs is extensive, varying greatly
in cost, effect on cholesterol fractions, efficacy, and side effects (Table 27.8).
Table 27.8. Drugs Used to Treat Hyperlipidemia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors (Statins)
These agents have become first-line drug therapy because of their effectiveness, patient acceptability, and
increasingly favorable safety record. They block the rate-limiting enzyme for cholesterol synthesis, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. This inhibition decreases intracellular cholesterol and increases clearance of LDL. Serum LDL levels fall by 20% to 60%, depending on dose and preparation. They affect plaque regression, even when used alone. HDL levels generally stay the same or increase slightly (2% to 10%). Statins also influence thrombotic and inflammatory mechanisms, effects whose importance remains to be proven but may account for some of the surprising CHD prevention efficacy seen in persons with lower LDL-cholesterol levels (see later discussion).
P.199
increasingly favorable safety record. They block the rate-limiting enzyme for cholesterol synthesis, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase. This inhibition decreases intracellular cholesterol and increases clearance of LDL. Serum LDL levels fall by 20% to 60%, depending on dose and preparation. They affect plaque regression, even when used alone. HDL levels generally stay the same or increase slightly (2% to 10%). Statins also influence thrombotic and inflammatory mechanisms, effects whose importance remains to be proven but may account for some of the surprising CHD prevention efficacy seen in persons with lower LDL-cholesterol levels (see later discussion).
Cost and Cost-Effectiveness
Cost and cost-effectiveness are important considerations
in selecting statin therapy because all these agents are expensive and
use is likely to be measured in years. Although fluvastatin has usually
been the least expensive and pravastatin and simvastatin the most
expensive of the statins, cost often depends on specific pricing
contracts with large payers, making generalizations difficult.
Lovastatin is now available as a generic agent, and simvastatin will
transition to that status in 2006, making it likely that some downward
cost pressure will be imposed on the whole class. The statins differ
principally in cost and potency, which often parallel one another. Cerivastatin
was removed from the market in the summer of 2001 because of high
adverse event report rates of myositis and rhabdomyolysis, particularly
when used in combination with gemfibrozil. Simvastatin, atorvastatin, and rosuvastatin
are the statins that give the greatest LDL-cholesterol reduction at
U.S. Food and Drug Administration (FDA)–approved dosing levels. Within
this group of three, the rank order of LDL-cholesterol–reducing
activity is simva < atorva < rosuva. LDL reductions of 50% to 65%
can be achieved with these drugs.
Although the major drawback to statin use is cost, cost
savings from reduced rates of cardiac events, time lost from productive
work, and premature death at least partly offset the high cost of
treatment. Formal cost-effectiveness analyses are appearing in the
literature. Data from the Scandinavian Simvastatin Survival Study
examined cost per year of life gained and found statin therapy
cost-effective in men and women over a broad spectrum of ages and
cholesterol ranges. The cost per year of life gained was less than half
of that for other cost-effective preventive measures such as
mammography.
Dosing
Starting dose for these agents is typically 10 to 20
mg/d, with the more potent drugs started at the lower dose. The maximum
dose is currently 80 mg/d for lovastatin, simvastatin, fluvastatin,
atorvastatin, and pravastatin. The maximum dose of rosuvastatin is 40
mg/d. The shorter-acting agents are best taken at night, the time of
peak cholesterol synthesis, but all drugs work satisfactorily even if
taken once daily in the morning.
Adverse Effects
Asymptomatic hepatocellular dysfunction
manifested by an increase in serum levels of hepatocellular enzymes
(e.g., aspartate and alanine aminotranferases) is among the most common
adverse effects. Incidence ranges from about 3% for any elevation to
less than 1% for elevations greater than three times the upper limit of
normal. All such increases are reversible with cessation of therapy,
which should be considered when liver enzyme levels continue to rise or
reach more than three times the upper limit of normal. Transaminase
monitoring is strongly recommended, with initial measurements made
after 1 to 2 months of therapy, and follow-up measurements made at 6
months and 1 year. The lack of new onset of liver toxicity after 1 year
of therapy has led many to advocate limited monitoring after 1 year.
Harmless elevations in muscle enzymes
(e.g., creatine phosphokinase <10 times the upper limit of normal)
occur in about 0.6% of cases and require no action in an asymptomatic
patient. Myalgias without creatine phosphokinase elevations also occur
with statin use, making routine monitoring of creatine phosphokinase of
limited value. Myalgias are probably underreported in patients on
statins, particularly in the elderly, because the symptom is frequently
attributed to arthritis or muscle stiffness associated with the aging
process. Patients should be questioned about increased muscle
discomfort when placed on statins, and drug holidays may need to be
instituted to clarify complaints. Symptomatic myositis
with demonstrable muscle breakdown is much less common, but the risk is
increased with high-dose therapy and when other lipid-lowering agents
are used in conjunction with statin therapy. Statin monotherapy has
been reported to cause rhabdomyolysis and renal failure, but it is more common in the setting of concurrent gemfibrozil
use. Dual therapy with niacin can also increase myositis. Joint use of
a statin and a fibrate (either gemfibrozil or fenofibrate) should be
done only with great caution and probably only by a lipid specialist.
Because of an FDA warning against this combination, pharmacists will
often not fill a prescription that places a patient on both drugs
without first calling the physician. The withdrawal of cerivastatin
from the market in 2001 was prompted by many reports of rhabdomyolysis
when the drug was used alone or, more commonly, in combination with a
fibrate. This event will likely increase the vigilance of pharmacists
in responding to combination-therapy prescriptions.
As noted earlier, initial concerns about an increase in
non-CHD mortality (e.g., from cancer, suicide, or violence) were raised
by early meta-analyses, but large-scale prospective studies of statins
show no evidence for such adverse effects.
Choice of Agent
Although most experts believe that all of the statins
will reduce coronary events in proportion to their efficacy in lowering
LDL cholesterol, not all statins have been proven to do so. The best
studies of clinical outcomes have been performed using simvastatin,
pravastatin, and lovastatin, but atorvastatin also has been found to
reduce cardiovascular event rates. Prospective studies with statins
have not only demonstrated a reduction in coronary events, but they
have also been shown to lower all-cause mortality. Patients who require
modest reductions in LDL cholesterol (25% or less) can be started on
any of the statins with the expectation that the reduction will be
achieved. Those with marked LDL elevations and high overall CHD risk
require more intensive therapy (>40% reductions) and are better
served if started on the more potent statins.
Niacin
This B vitamin contributes to dyslipidemia therapy by virtue of its unique ability to markedly raise HDL cholesterol
(on the order of 15% to 35%). Its purported mechanism of action
involves inhibiting mobilization of free fatty acids from fat tissue to
the liver. The substance also lowers triglycerides (by 20% to 50%) and LDL cholesterol
(by 5% to 25%), converting small LDL-cholesterol particles to more
bouyant less atherogenic forms. Use in high-risk persons (e.g., prior
myocardial infarction) has been associated with significant reductions
in new myocardial infarction (26%), stroke (24%), and death (11%).
Niacin in combination with colestipol or a statin has produced documented regression of atheromatous plaque in coronary
arteries. Although effective, niacin therapy often requires high doses (1.5 to 3.0 g/d) to achieve the desired results. Use in combination with a statin (which can also raise HDL cholesterol) may allow for somewhat lower doses.
P.200
arteries. Although effective, niacin therapy often requires high doses (1.5 to 3.0 g/d) to achieve the desired results. Use in combination with a statin (which can also raise HDL cholesterol) may allow for somewhat lower doses.
Adverse Effects
A high-capacity, early route of niacin metabolism involves conjugation with glycine to produce nicotinuric acid, which produces flushing,
the immediate and principle side effect that limits clinical use.
Tolerance to flushing develops over time and allows slow advancement of
dose. The bothersome side effect can also be lessened by starting with
a low dose (e.g., 100 mg thrice daily), using an extended-release
preparation (see later discussion), taking once-daily pretreatment with
a nonsteroidal antiinflammatory drug (e.g., aspirin 325 mg, or
ibuprofen 200 mg, 30 to 30 minutes before first dose), and taking
niacin with meals.
Hepatotoxicity is the other
major concern, related to nonconjugative metabolism of the drug to
nicotinamide and pyrimidines. Risk of hepatotoxicity is greatest with
use of sustained-release preparations (18
to 24 hours' duration), which are metabolized predominantly by the
nonconjugative route. Risk is minimized by taking an intermediate
(“extended”)-release formulation (8 to 10 hours' duration), which
optimizes metabolism through both pathways. Periodic monitoring of
liver function (e.g., aspartate aminotransferase determination) is
required with niacin use.
Other adverse effects include exacerbation of gout and slight worsening of glucose intolerance, necessitating an occasional check of uric acid and glucose. Rashes, dry skin, and occasionally acanthosis nigricans
may accompany niacin use. Lanolin cream helps the former, and prompt
cessation clears the latter. Some patients experience gastrointestinal
upset.
Preparations
As a B-complex vitamin (nicotinic acid), niacin is available without prescription in immediate-release and sustained-release
forms. Although least costly, these are associated with the highest
frequency of flushing and hepatotoxicity, respectively. A prescription
is required for the intermediate-release preparation (also referred to as extended-release
niacin [e.g., Niaspan]), which is the best tolerated but most expensive
formulation. Its use should be considered in persons who cannot
tolerate the immediate-release preparation or in whom there is concern
about risk of hepatoxicity. If an inexpensive, well-tolerated,
immediate-release brand of niacin is found, the patient should stay
with it; changing brands might not be as well tolerated.
Bile Acid Sequestrants (Cholestyramine, Colestipol, Colsevelam)
These nonabsorbable agents have been first-line
pharmacologic therapy for many years, with an established record of
safety. They are very useful for patients who are not at great CHD risk
but in whom diet alone fails to lower LDL cholesterol to target levels.
They are also of benefit in individuals who are statin intolerant.
Though not as cost-effective as the statins or niacin, they are very
effective when used in combination with them to treat high-risk
patients with severe hypercholesterolemia. The sequestrants bind bile
acids in the gut and interrupt their normal enterohepatic circulation.
The resultant shunting of cholesterol in the liver to bile acid
production leads to a fall in total- and LDL-cholesterol levels.
However, triglyceride levels often rise with this class of drugs,
particularly if moderate hypertriglyceridemia antedates the initiation
of the therapy. Cholestyramine and colestipol are prescribed as powders
that are dissolved in liquid. Colesevelam, which comes as a 625-mg
pill, also binds biliary lipids in the gastrointestinal tract and has
comparable effects on the serum cholesterol.
Adverse Effects
The bile sequestrants are nonabsorbable resins whose major side effects are gastrointestinal—constipation, bloating, heartburn, and nausea.
A high-fiber diet or psyllium supplement and use of these agents just
before a meal will usually ameliorate the gastrointestinal upset. The
potential to impede absorption of certain drugs
(e.g., digoxin, thyroxin, warfarin, tetracycline, phenobarbital)
necessitates that bile sequestrants not be taken until at least 1 hour
after or 4 hours before these other drugs. In rare instances,
steatorrhea and malabsorption of the fat-soluble vitamins (A, D, E, and
K) can occur. The usual starting dose is one scoop of the powdered form
of the drug (4 g of cholestyramine, 5 g of colestipol) in a large glass
of water twice a day. Dose can be increased to a total of three scoops
twice daily. A newer formulation of cholestyramine, which is
significantly less gritty (Lo-Cholest), can be tried if the texture of
the generic drug formulation should prove unacceptable. Colesevelam is
given as six 625-mg tablets a day, given in once-a-day or twice-daily
dosing.
Ezetimibe
Ezetimibe is a new agent that, like the bile acid
sequestrants, blocks cholesterol absorption from the gut. Unlike the
sequestrants, however, ezetimibe is postulated to inhibit cholesterol
transport by interfering with a specific transporter protein required
for this activity rather than interfering with micellar solubization of
lipid. This difference permits ezetimibe to be given in milligram doses
rather than gram doses, and it does not interfere with the absorption
of other drugs and fat-soluble vitamins. The ease of use and
tolerability of ezetimibe have led to its use in many of the
circumstances where bile acid sequestrants were formerly used. As a
single agent, ezetimibe lowers LDL cholesterol by 15% to 20%. When used
in conjunction with a statin, it typically provides an additional 15%
reduction in the LDL-cholesterol level.
Estrogens
In postmenopausal women, estrogen replacement therapy is effective in lowering the levels of LDL cholesterol and raising those of HDL cholesterol.
These changes would be expected to reduce coronary events, and
retrospective analyses of hormonal replacement therapy initially
confirmed this expectation, but several large-scale, prospective,
randomized trials of estrogen therapy or estrogen plus progestin failed
to demonstrate any benefit in reducing cardiovascular morbidity or
mortality. The Women's Health Initiative study was prematurely ended in
2002 when the rate of invasive breast cancer in the
estrogen–progestin-treated group exceeded the stopping boundary for
this condition. No benefit on cardiovascular status was seen in the
hormone-treated cohort. The estrogen-only arm of the same trial was
terminated in 2004, again with no benefit on CHD morbidity or mortality
detected. Thus, despite the beneficial impact on serum lipids, hormone
replacement therapy has failed to produce improved cardiovascular
outcomes and can no longer be recommended as a standard therapy for the
prevention of CHD.
Fibrates (Gemfibrozil and Fenofibrate)
Because these drugs lower LDL cholesterol much less
effectively than the statins, they are not considered first-line drugs
for the treatment of hypercholesterolemia. Nonetheless, they do have
specific uses. They decrease VLDL synthesis and enhance its clearance. They also raise HDL cholesterol, most prominently in
those who have concomitant triglyceride elevations. The effect on LDL cholesterol is variable, although an 8% to 15% reduction can be seen in patients who do not have markedly elevated VLDL levels.
P.201
those who have concomitant triglyceride elevations. The effect on LDL cholesterol is variable, although an 8% to 15% reduction can be seen in patients who do not have markedly elevated VLDL levels.
Both fibrate agents are generally well tolerated, but
they can increase bile cholesterol content, raising the risk of
gallstone formation and potentiating the effect of warfarin. The FDA
has issued a warning about their use in combination with statins
because rhabdomyolysis has occurred when
gemfibrozil and a statin are used concurrently. An earlier drug in this
class, clofibrate, was taken off the market because of reports of increased mortality
associated with its use. Although the FDA has approved both fibrates,
these agents have yet to demonstrate a reduction in all-cause
mortality. A Veterans Administration gemfibrozil study of men with low
HDL cholesterol did show improvement in coronary outcomes; HDL
cholesterol rose, triglycerides fell, and LDL cholesterol stayed the
same.
Pharmacologic Therapy: Treatment Thresholds, Goals, and Monitoring (2,5,9,17,20,26,35)
Treatment Thresholds
Current guidelines derive from the evidence-based
consensus recommendations of the NCEP panel and reflect the trend
toward lower LDL-cholesterol thresholds for treatment in persons at
increased risk. Recent major clinical trials have shown improved
outcomes using low LDL cut-points.
For Those at Very High Risk (e.g., Acute Coronary Insufficiency or CHD plus Multiple Severe Risk Factors)
The newest revisions recommend an optional LDL-cholesterol treatment threshold of 100 mg/dL, with a goal of less than 70 mg/dL.
For Those at Moderately High Risk (No CHD, but Multiple Risk Factors and 10-Year CHD Risk of 10% to 20%)
The LDL-cholesterol treatment threshold has been lowered to 130 mg/dL, with a treatment goal of less than 100 mg/dL.
For Those at Moderate Risk with Two or More CHD Risk Factors (Two or More Risk Factors, 10-Year Risk Probability Is <10%)
The threshold for persons in the lower-risk categories
remains unchanged from previous recommendations. For this group the
LDL-cholesterol cut-point continues to be 160 mg/dL.
For Those with Fewer Than Two CHD Risk Factors
Drug therapy is definitively recommended for LDL-cholesterol levels above 190 mg/dL and considered optional for those with values between 160 and 190 mg/dL.
For Those with Isolated Low HDL Cholesterol
Although epidemiologic data show a strong inverse
relation between HDL level and CHD risk, there are no data yet from
large-scale, randomized, prospective clinical trials showing that
raising HDL cholesterol alone significantly reduces CHD mortality.
However, treatment of patients with low HDL levels with statins does
seem to lower CHD morbidity. Generally, healthy middle-aged and elderly
persons with low HDL cholesterol and “normal” LDL cholesterol
demonstrate a significant reduction in risk of a first acute major
coronary event (e.g., myocardial infarction, unstable angina) when
treated with a statin drug (e.g., the AFCAPS/TexCAPS trial). Such
findings suggest that even persons with modest increases in CHD risk—as
manifested by advancing age and an isolated low HDL cholesterol—might
benefit from pharmacologic therapy. The mechanism of the benefit may be
other than the effect on HDL cholesterol because in the AFCAPS/TexCAPS
study, HDL cholesterol rose only 6%.
Effect of Threshold on Cost-Effectiveness
Lowering the threshold for drug therapy below that
recommended by the new NCEP guidelines could be justified on the basis
of recent outcome studies, but the cost-effectiveness of such an
approach remains to be established. Because results are typically
reported in terms of reduction in relative risk, the magnitude of the
benefit to lower-risk patients may sometimes appear inflated. Absolute
risk certainly becomes an issue in younger patient populations, where a
20% to 30% reduction in relative risk may represent only a modest
clinical achievement (i.e., if the absolute risk of having a CHD event
over the next 10 years is only 2%, a 20% reduction results in the
absolute risk falling to 1.6%).
Treatment Goals
The ultimate treatment goal is reduction in CHD risk; the immediate one is reduction of LDL cholesterol. Target levels have been lowered, reflecting the improvement in outcomes associated with lower LDL-cholesterol levels. For primary prevention (no established CHD), the NCEP target is an LDL-cholesterol level less than 130 mg/dL, although ATP III now defines an optimal LDL cholesterol as less than 100 mg/dL and sets an optional LDL cholesterol target of less than 100 mg/dL for persons with moderately high CHD risk (estimated 10-year risk 10% to 20%). For secondary prevention (established CHD), the goal is an LDL-cholesterol level less than 100 mg/dL with an optional goal of less than 70 mg/dL for persons at very high risk.
The latest NCEP guidelines introduced the concept of elevated non-HDL-cholesterol
levels. Non-HDL cholesterol is determined by subtracting the
HDL-cholesterol value from the total-cholesterol value. Patients with
high triglyceride levels will have elevations in their VLDL
cholesterol, which in turn contributes to a higher non-HDL cholesterol
level. ATP III suggests for patients with high triglyceride levels
substituting a non-HDL-cholesterol goal in place of the LDL-cholesterol
goal and setting the cut-point 30 mg/dL higher than the LDL cut-point.
The rationale is that the calculated LDL may not be accurate in
patients with high triglycerides. This 30-point differential derives
from the Friedewald formula, where VLDL cholesterol is represented by
the triglyceride value divided by 5 (i.e., 150/5 = 30). So, as the
triglyceride level rises above 150 mg/dL, the VLDL contribution to the
non-HDL cholesterol will rise above 30 mg/dL.
Monitoring
This is performed by measurement of the LDL-cholesterol
level, beginning about 6 to 8 weeks after initiation of therapy and
then every 3 to 4 months until control is established. Afterward, every
6 to 12 months is usually sufficient. More-frequent monitoring for
development of abnormalities in serum chemistries (e.g., liver enzymes)
is indicated when using certain pharmacologic agents (see prior
discussion).
Treatment in the Elderly (9,16)
Prevalence of hypercholesterolemia is greatest in those
older than 65 years of age. As in other age groups, elevations in total
and LDL cholesterol are predictive of increased cardiovascular risk.
However, the statistical risk relationship is not as strong as in
younger patients due in part to the frequent occurrence of other
important risk factors in the elderly (e.g., diabetes, hypertension).
Elderly patients may have other advanced diseases, making prevention of
coronary disease appear irrelevant to their overall quality of life. In
those who are vigorous and have a considerable life expectancy,
however, primary and secondary prevention of CHD can be very important.
P.202
Several factors favor treatment. Life expectancy
continues to lengthen, and the quality of life in the elderly also has
steadily improved. Treating individuals in their late 60s and 70s with
statins has been shown to reduce CHD and stroke, two of the major
causes of mortality in that age group. The elderly are more likely to
have preexisting CHD, and the benefits on secondary prevention of
coronary disease by lowering cholesterol exceed the benefits of primary
prevention. With the advent of better tolerated cholesterol-lowering
medications, the risks of adverse effects and their negative impact on
quality of life have declined. All these factors combine to make the
recommendation to treat hypercholesterolemia in the elderly quite
appropriate.
Dietary Measures
Dietary therapy of hypercholesterolemia in the elderly
is similar to that for all adults and should be carried out as the
first step in treatment, though dietary measures do not always suffice
by themselves. Carbohydrate should not replace most fat in the diet,
rather polyunsaturates and monounsaturates should be increased. The Ω-6
polyunsaturated fatty acids found in vegetable oils should not exceed
10% of calories. For the elderly, modifications of the usual
low-saturated-fat diet are needed to ensure adequate calcium intake for
prevention of osteoporosis. Use of skim milk and low-fat and nonfat
yogurts are examples of ways to maintain calcium intake while cutting down on saturated fat. Maintaining adequate protein intake
is also essential, meaning that lean cuts of red meat ought to be
allowed in addition to fish and skinless chicken to ensure palatability
of the diet. High fiber is essential for good bowel function and cannot hurt the cholesterol-lowering effort.
Pharmacologic Therapy
Because dietary therapy alone frequently fails to
achieve the goal of an LDL cholesterol lower than 130 mg/dL, drug
treatment must often be considered.
Statin therapy is indicated
when aggressive lowering of LDL cholesterol is needed. The statins have
also proven useful for primary prevention in elderly persons with low
HDL cholesterol and average LDL cholesterol. These drugs are well
tolerated in the elderly, with minor diarrhea, myalgias, and occasional
sleep disturbances being the most common problems. Minor transaminase
elevations are common; they are usually asymptomatic and not a cause
for discontinuation unless they exceed three-times normal. However,
regular transaminase monitoring is required throughout the course of
therapy. As noted earlier, initial concerns about an increased risk of
malignancy have proven unfounded in large-scale, prospective, long-term
follow-up studies. Statins are recommended as first-line drug therapy
for hypercholesterolemia in the elderly.
The bile sequestrants are
safe but can cause considerable gastrointestinal upset, especially
constipation. Increasing dietary fiber helps. Because sequestrants can
impair drug absorption, their use in elderly patients must include
instruction to take other medications at least 1 hour before or 4 hours
after sequestrant use. Among the drugs that might be affected by
sequestrants are warfarin, propranolol, digitalis preparations,
thyroxin, and antibiotics. Although low-dose sequestrants are a
reasonable first choice for pharmacologic therapy, the availability of
ezetimibe has provided a better tolerated alternative to statins.
Niacin is effective,
although not always well tolerated. Its advantages over statins are its
ability to also raise HDL cholesterol and its low cost. The incidence
of side effects in the elderly is high, with flushing, gastrointestinal
upset, dry mouth, and dry eyes being particularly annoying. The drug
may exacerbate peptic ulcer disease, elevate transaminases, and trigger
arrhythmias and hypotension. Multiple daily doses are usually required
if the nonprescription forms are used. The once-daily formulation of
niacin, Niaspan, has some distinct advantages but is associated with a
higher cost than the nonprescription formulations.
PATIENT EDUCATION (6,9,25)
Regarding Diet and Exercise
The importance of patient education in the management of
hyperlipidemia cannot be overemphasized because treatment starts with
alterations in the patient's eating and exercise habits (see Chapters 18 and 233).
The first step in therapy should be a careful review of the rationale
for treating hypercholesterolemia, followed by a discussion of basic
dietary principles for lowering cholesterol. Consultation with a
dietitian can be very helpful. Many patients are surprised to learn
that dietary fat is more atherogenic than dietary cholesterol itself
(witness the patient who eats cholesterol-free potato chips with
abandon). Reviewing the saturated fat and trans
fat content of foods regularly consumed by the patient is quite
worthwhile. At times, simply removing a few grossly offending foods
from the diet (e.g., processed snack foods, cheese, grossly fatty
meats, cold cuts, fried food) will ensure a good start to a change in
eating habits. More comprehensive diet planning can be aided by
discussion with the nurse or dietitian, facilitated by written material
such as that produced by the American Heart Association. Periodic
visits to check diet, weight, and cholesterol are excellent, although
often overlooked, means of facilitating compliance and providing
reinforcement.
Regarding Medications
Patients need to understand the rationale for their
medical program and the details of its proper use and side effects.
Some mistakenly believe their drug program is curative and stop
treatment after a few months of therapy. Others harbor exaggerated
concerns about adverse drug effects and stop medication prematurely.
Cost is another factor that often limits compliance, necessitating a
strategy that takes into account the patient's insurance coverage for
medication. However, careful studies find that adequacy of insurance
does not by itself explain the nearly 40% fall-off in compliance that
occurs over 5 years among patients prescribed lipid-lowering
medication. Choice of agent, comorbidity, and socioeconomic status also
play important roles, underscoring the importance of comprehensive and
ongoing patient education.
INDICATIONS FOR REFERRAL
To the Dietician
Patients prescribed a dietary program should have a consultation with a dietitian
if they are unclear about the food choices they should make or if
compliance with the diet is problematic. Dieticians can provide
educational materials, food preparation advice, and the periodic
feedback that patients often need to permanently change their eating
habits.
To the Lipid Specialist
Patients with high-risk lipid profiles who do not
respond to diet plus one or two first-line drugs, those with extremes
of any lipoprotein level, or a family history of premature coronary
disease (before age 55 years) should be considered for referral to a
physician expert in diagnosis
and treatment of lipid disorders. Some genetic disorders are particularly refractory to standard therapies, and patients with these conditions will benefit from consideration of genetic links and more complex treatment regimens that are the province of a lipid specialist. Identification of affected family members can also be accomplished. Lipid research laboratories can often categorize specific genetic abnormalities through testing not routinely available in most clinical laboratories. Ultracentrifugation of lipoproteins, polymerase chain reaction amplification of DNA, and cell-based receptor and enzymatic assays can be used to help pinpoint the cause and screen other family members for the problem. Although these more sophisticated tests may not yet translate into different therapeutic options, they often help clarify family questions about the risks of CHD in related individuals and are likely to influence therapeutic options in the years ahead.
P.203
and treatment of lipid disorders. Some genetic disorders are particularly refractory to standard therapies, and patients with these conditions will benefit from consideration of genetic links and more complex treatment regimens that are the province of a lipid specialist. Identification of affected family members can also be accomplished. Lipid research laboratories can often categorize specific genetic abnormalities through testing not routinely available in most clinical laboratories. Ultracentrifugation of lipoproteins, polymerase chain reaction amplification of DNA, and cell-based receptor and enzymatic assays can be used to help pinpoint the cause and screen other family members for the problem. Although these more sophisticated tests may not yet translate into different therapeutic options, they often help clarify family questions about the risks of CHD in related individuals and are likely to influence therapeutic options in the years ahead.
THERAPEUTIC RECOMMENDATIONS (9,26,42)
Effective reduction of CHD risk requires identifying and
aggressively treating all CHD risk factors responsive to medical
intervention, including smoking, hypertension, diabetes, and obesity
(see Chapters 26, 54, 102, and 233). Focusing on hyperlipidemia alone is insufficient. In treating hypercholesterolemia, one should determine total CHD risk (see Tables 27.4 and 27.5) and treat accordingly (see Table 27.6).
- Prescribe dietary restriction of total fat intake to no more than 20% of calories, substituting polyunsaturated and monounsaturated fats for saturated fat, partially hydrogenated unsaturated fat, and cholesterol. Consider use of phase II or phase III dietary programs to achieve LDL reductions of 40 to 80 mg/dL and minimize intensity of necessary pharmacologic therapy.
- Initiate and maintain therapeutic lifestyle changes in those with a lifestyle-related risk factor (e.g., obesity, physical inactivity, metabolic syndrome, low HDL cholesterol, elevated triglycerides), regardless of LDL-cholesterol level, and in those with an LDL cholesterol greater than 100 mg/dL.
- Initiate pharmacologic therapy with a statin preparation at the same time or shortly after implementing dietary and lifestyle measures if the LDL cholesterol is greater than 130 mg/dL. In very-high-risk persons—that is, established cardiovascular disease plus (a) acute coronary syndrome, (b) multiple major risk factors including diabetes mellitus, (c) severe and poorly controlled risk factors (especially smoking), or (d) metabolic syndrome— consider statin therapy even if the LDL cholesterol is less than 100 mg/dL but greater than 70 mg/dL; use clinical judgment in such circumstances to balance pharmacologic risk with benefit.
- Treat intensively enough to reduce LDL cholesterol by at least 30% to 40%. Aim for an LDL cholesterol of less than 100 mg/dL and for less than 70 mg/dL in those at very high risk.
- Minimize cost of statin therapy by matching statin preparation with degree of LDL-cholesterol reduction needed. For example, if an LDL-cholesterol reduction of greater than 35% is required, consider starting simvastatin 20 mg/d, atorvastatin 10 mg/d, or rosuvastatin 5 to 10 mg/d (see prior discussion); if an LDL-cholesterol reduction of less than 35% is needed, then almost any statin can be used, and cost should determine choice.
- Consider adding an extended-release niacin preparation (beginning with 500 to 1,000 mg/d) if LDL-cholesterol elevation is accompanied by a low HDL cholesterol (<40 mg/dL).
- Consider adding a second agent in those who fail to adequately respond to diet plus maximal doses of a single agent. The bile resins (e.g., cholestyramine or colestipol, one to two scoops twice daily) or ezetimibe are well suited to combination programs. One should usually avoid the combination of a statin plus gemfibrozil because of the increased risk of rhabdomyolysis and the combination of a statin plus full doses of niacin because of increased risk of myositis.
Moderate Risk (LDL Cholesterol 130 to 159 mg/dL plus
Two CHD Risk Factors or LDL Cholesterol 160 to 189 mg/dL and No Other
CHD Risk Factors)
- Begin with dietary therapy. In patients with large intakes of saturated fat and cholesterol, the phase I diet can produce sufficient reductions in cholesterol of 20 to 40 mg/dL. More aggressive dietary fat restriction (phase II diet) may be needed in those who do not respond adequately. PUFAs should be increased moderately, but to no more than 10% of total calories.
- Implement and maintain therapeutic lifestyle changes regardless of LDL-cholesterol level in those with a lifestyle-related risk factor (e.g., obesity, physical inactivity, metabolic syndrome, low HDL cholesterol, elevated triglycerides) and in those with an LDL cholesterol greater than 130 mg/dL.
- Add pharmacologic therapy if LDL cholesterol is greater than 130 mg/dL after 4 to 6 months of dietary and lifestyle changes. Consider in those at moderately high risk (calculated 10-year cardiovascular risk of 10% to 20%) an optional pharmacologic treatment threshold of between 100 and 129 mg/dL.
- Begin with a statin, and treat with sufficient intensity to achieve at least an additional 30% to 40% reduction in LDL cholesterol.
- Aim for a minimum goal of reducing LDL cholesterol to less than 130 mg/dL, and in those at moderately high risk, consider an optional LDL-cholesterol goal of less than 100 mg/dL.
Modest Risk (Isolated Low HDL Cholesterol [<40 mg/dL]; LDL Cholesterol Not Elevated)
- Prescribe nonpharmacologic measures that can increase HDL cholesterol, including aerobic exercise, smoking cessation, and weight loss if obese. Such actions can increase HDL by 5 to 15 mg/dL.
- Prescribe a phase I diet, in which saturated fat, cholesterol, and partially hydrogenated vegetable oils are restricted and replaced by monounsaturated and polyunsaturated fats.
- Use clinical judgment in considering pharmacologic treatment if nonpharmacologic measures prove insufficient.
- Although the NCEP does not yet recommend pharmacologic correction of an isolated HDL cholesterol, pay attention to total CHD risk. In those with multiple cardiac risk factors and a low HDL cholesterol consider statin therapy for middle-aged and elderly persons; consider niacin as an additional option, especially for younger persons, who tolerate the drug better than do the elderly; always take into account HDL-cholesterol level when designing a pharmacologic program for persons with LDL-cholesterol elevation.
P.204
Elevated Triglycerides (Fasting Triglycerides >200 mg/dL)
- Because no consensus exists on need for treatment, exercise clinical judgment.
- Consider treatment in persons with elevated triglycerides in the setting of low HDL cholesterol (<40 mg/dL), because the latter may rise substantially with use of triglyceride-lowering drugs.
- To accomplish this goal, consider gemfibrozil (600 mg twice daily) or fenofibrate (45–145 mg/d).
- Such drugs can also be used to reduce the risk of pancreatitis in persons with very high triglyceride levels (>800 mg/dL).
- Substitute a non-HDL-cholesterol level as the goal in place of the LDL-cholesterol, setting the cut-point 30 mg/dL higher than the LDL cut-point.
All Patients
- Fully explain the condition and the rationale for the treatment program to ensure compliance; emphasize the importance of dietary modification, exercise, and compliance with any drug program that might be necessary. Customize the patient education and treatment programs to the needs and capabilities of the patient.
- Address patient concerns, especially those regarding long-term use of lipid-lowering medications or dietary modifications. Enlist the services of a dietician if there are concerns or questions regarding dietary changes.
- Consider for referral to a lipid specialist patients with high-risk lipid profiles who do not respond to diet plus one or two first-line drugs, those with extremes of any lipoprotein level, or a family history of premature coronary disease (before age 55 years).
ANNOTATED BIBLIOGRAPHY
1. Criqui
MH, Heiss G, Cohn R, et al. Plasma triglyceride level and mortality
from coronary heart disease. N Engl J Med 1993;328:1220. (No
independent association with coronary heart disease [CHD] risk was
found, but its relation to low high-density lipoprotein was noted.)
2. Heart
Protection Study Collaborative Group. MRC/BHF Heart Protection Study of
cholesterol-lowering with simvastatin in 5963 people with diabetes: a
randomised placebo-controlled trial. Lancet 2003;361:2005. (Establishes benefit of lipid lowering in diabetics and underscores the contribution of diabetes to CHD risk.)
3. Hu
FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of
coronary heart disease in women. N Engl J Med 1997;337:1491. (Major
prospective epidemiologic study; finds that replacing saturated and
trans unsaturated fats with polyunsaturated fat produced a greater
reduction in coronary disease risk than did reducing overall fat intake.)
4. Jenkins
DJA, Uolever TMS, Venketshwer R, et al. Effect on blood lipids of very
high intakes of fiber in diets low in saturated fat and cholesterol. N
Engl J Med 1993;329:21. (Foods rich in soluble fiber can lower cholesterol.)
5. Klag
KJ, Ford DE, Mead LA, et al. Serum cholesterol in young men and
subsequent cardiovascular disease. N Engl J Med 1993;328:313. (Strong association was found between serum cholesterol level in early life and CHD in midlife.)
6. Mensink RP, Katan M. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1991;323:439. (Partially
hydrogenated unsaturated fatty acids of processed foods were found to
promote undesirable lipid levels as much as or more than the saturated
fatty acids they were intended to replace.)
7. The
Lipid Research Clinics Coronary Primary Prevention Trial results. I.
Reduction in incidence of coronary heart disease. JAMA 1984;251:351. (Early prospective data establishing the effect of lipid lowering on CHD risk.)
8. The
Lipid Research Clinics Coronary Primary Prevention Trial results. II.
The relationship of reduction in incidence of coronary heart disease to
cholesterol lowering. JAMA. 1984;251:351,365. (Early prospective data documenting the reduction in risk of CHD.)
9. Expert
Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol
in Adults (Adult Treatment Panel III). Executive summary of the third
report of the National Cholesterol Education Program (NCEP) expert
panel on detection, evaluation, and treatment of high blood cholesterol
in adults. JAMA 2001;285:2486. (Major consensus recommendations.)
10. Danesh
J, Wheeler J, Hirschfield G, et al. C-reactive protein and other
circulating markers of inflammation in the prediction of coronary heart
disease. N Engl J Med 2004;350:1387. (Data suggesting that the degree of risk is less than originally suspected.)
11. Eikelboom
JW, Lonn E, Genest J Jr, et al. Homocyst(e)ine and cardiovascular
disease: a critical review of the epidemiologic evidence. Ann Intern
Med 1999;131:363. (A systematic review finding strong epidemiologic evidence for an association but no proof that treatment lowers CHD risk.)
12. Nygard
O, Nordrehaug JE, Refsum H, et al. Plasma homocysteine levels and
mortality in patients with coronary artery disease. N Engl J Med
1997;337:230. (Some of the strongest
epidemiologic data suggesting that homocysteine elevation is a potent
and independent predictor of mortality.)
13. Ridker
PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and
low-density lipoprotein cholesterol levels in the prediction of first
cardiovascular events. N Engl J Med 2002;347:1557. (Evidence
for that C-reactive protein was an independent risk factor and possibly
of the same magnitude as that for low-density-lipoprotein [LDL]
cholesterol; see also ref. 10.)
14. Ridker,
PM, Rifai N, Clearfield M, et al. Measurement of C-reactive protein for
the targeting of statin therapy in the primary prevention of acute
coronary events. N Engl J Med 2001;344:1959. (Case for the use of C-reactive protein to help guide treatment.)
15. Cannon
CP, Braunwald E, McCabe CH, et al. Comparison of intensive and moderate
lipid lowering with statins after acute coronary syndromes. N Engl J
Med 2004;350:1495. (Randomized,
controlled trial [RCT] in very-high-risk patients; secondary prevention
with intensive therapy was superior to moderate lipid lowering.)
16. Denke MA, Grundy SM. Hypercholesterolemia in the elderly: resolving the treatment dilemma. Ann Intern Med 1990;112:780. (A detailed review of the risks and benefits; favors treatment of high-risk patients; 83 references.)
17. Nissen
SE, Tuzcu EM, Schoenhagen P, et al. Effect of intensive compared with
moderate lipid-lowering therapy on progression of coronary
atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071. (Finds intensive lipid lowering prevented the progression of atherosclerosis.)
18. Brown
BG, Zhao ZQ, Chait A, et al. Simvastatin and niacin, antioxidant
vitamins, or the combination for the prevention of coronary disease. N
Engl J Med 2001;345:1583. (RCT; there was no added benefit from antioxidant vitamins.)
19. Heart
Protection Study Collaborative Group. MRC/BHF Heart Protection Study of
antioxidant vitamin supplementation in 20,536 high-risk individuals: a
randomised placebo-controlled trial. Lancet 2002;360:23. (Large RCT with 5-year follow-up; no benefit was demonstrated from vitamins C, E, and beta-carotene.)
P.205
20. Hunninghake
DB, Stein EA, Dujovne CA, et al. The efficacy of intensive dietary
therapy alone or combined with lovastatin in outpatients with
hypercholesterolemia. N Engl J Med 1993;328:1213. (Diet alone provided only a 5% LDL reduction; medication added another 27%.)
21. Isaacsohn
JL, Moser M, Stein EA, et al. Garlic powder and plasma lipids and
lipoproteins: a multicenter, randomized, placebo-controlled trial. Arch
Intern Med 1998;158:1189. (Garlic
powder was given at a dose of 900 mg/d for 12 weeks; no significant
differences were noted compared with the group prescribed placebo.)
22. Pearson
TA, Patel RV. The quest for a cholesterol-decreasing diet. Should we
subtract, substitute, or supplement. Ann Intern Med 1993;119:627. (An editorial arguing for reduction in fat intake as the primary therapy; a good review of dietary supplements.)
23. Schaefer
EJ, Lamon-Fava S, Ausman LM, et al. Individual variability in
lipoprotein cholesterol response to NCEP step 2 diets. Am J Clin Nutr
1997;65:823–830. (A metabolic ward
study of a step 2 diet showing an impressive reduction in serum
cholesterol levels in the whole study group on average, but the
variability among individuals was quite substantial.)
24. Sprecher
DL, Harris BV, Goldberg AC, et al. Efficacy of psyllium in reducing
serum cholesterol levels in hypercholesterolemic patients on high- or
low-fat diets. Ann Intern Med 1993;119:545. (A 5% to 10% reduction was achieved in both groups.)
25. Stern
L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus
conventional weight loss diets in severely obese adults: one-year
follow-up of a randomized trial. Ann Intern Med 2004;140:778. (Triglycerides were reduced, glucose was intolerance improved; there was no major change in LDL cholesterol.)
26. Topol EJ. Intensive statin therapy — a sea change in cardiovascular prevention. N Engl J Med 2004;350:1562. (An editorial summarizing the data in support of intensive therapy and lower LDL-cholesterol target levels.)
27. Shepherd
J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with
pravastatin in men with hypercholesterolemia. N Engl J Med
1995;333:1301. (RCT, the West of
Scotland Study; finds approximately 30% reductions in rates of nonfatal
infarction, CHD death, and cardiovascular death in men without CHD but
with multiple risk factors.)
28. Downs
JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary
events with lovastatin in men and women with average cholesterol
levels: results of AFCAPS/TexCAPS. JAMA 1998;279:1615. (Major
RCT on primary prevention; healthy middle-aged and elderly patients
with low HDL and normal LDL; there was a 37% reduction in CHD risk
demonstrated over 5 years of follow-up.)
29. Frick
MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention
trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of
treatment, changes in risk factors, and incidence of coronary heart
disease. N Engl J Med 1987;317:1237. (Original major study showing benefit for this class of drugs.)
30. Grodstein F, Stampfer MJ, Colditz GA, et al. Postmenopausal hormone therapy and mortality. N Engl J Med 1997;336:1769. (Original epidemiologic data suggesting a lower risk of death; later contradicted by prospective, randomized trials.)
31. Heart
Protection Collaborative Study Group. MRC/BHF Heart Protection Study of
cholesterol lowering with simvastatin in 20,536 high-risk individuals:
a randomised placebo-controlled trial. Lancet 2002;360:7. (Major RCT; shows a significant lowering of risk in high-risk persons, including those with diabetes.)
32. Henkin
Y, Oberman A, Hurst DC, et al. Niacin revisited: clinical observations
on an important but underutilized drug. Am J Med 1991;91:239. (Makes the case for more use of this effective, inexpensive drug but notes dose-related toxicities.)
33. Hulley
S, Grady S, Bush T, et al. Randomized trial of estrogen plus progestin
for secondary prevention of coronary heart disease in postmenopausal
women. JAMA 1998;280:605. (RCT; there was no benefit of hormone replacement therapy on survival in women with established coronary disease.)
34. Johannesson
M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin
treatment to lower cholesterol levels in patients with coronary heart
disease. N Engl J Med 1997;336:332. (Data
from the Scandanavian Simvastatin Survival Study showing simvastatin
treatment is cost-effective in men and women over a wide range of ages
and cholesterol elevations.)
35. LIPID
Study. Prevention of cardiovascular events and death with pravastatin
in patients with coronary heart disease and a broad range of initial
cholesterol levels. The Long-Term Intervention with Pravastatin in
Ischaemic Disease Study Group. N Engl J Med 1998;339:1349. (RCT;
treatment of modestly elevated LDL cholesterol levels improved
cardiovascular mortality by 24%, and overall mortality was reduced 22%.
No increase in breast cancer was noted.)
36. Pierce
LR, Wysowski DK, Gross TP. Myopathy and rhabdomyolysis associated with
lovastatin–gemfibrozil combination therapy. JAMA 1990;264:71. (Documents this serious complication; the combination is generally to be avoided.)
37. Pitt
B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy
compared to angioplasty in stable coronary artery disease. N Engl J Med
1999;341:70. (Atorvastatin was at least as effective as angioplasty for reducing the rate of coronary events.)
38. Rossouw,
JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen
plus progestin in healthy postmenopausal women: principal results from
the Women's Health Initiative randomized controlled trial. JAMA
2002;288:321. (No cardiovascular benefit found.)
39. Rubins
HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary
prevention of coronary heart disease in men with low levels of
high-density lipoprotein cholesterol. N Engl J Med 1999;341:410. (RCT; the Veterans Affairs High-Density Lipoprotion trial; reductions in risks of infarction and death were demonstrated.)
40. Sacks
FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary
events after myocardial infarction in patients with average cholesterol
levels. N Engl J Med 1996;335:1001. (An
important extension of the Scandinavian Simvastatin Study in that
patients with much lower LDL-cholesterol values were chosen for
cholesterol lowering; improved cardiovascular mortality was noted.)
41. Scandinavian
Simvastatin Survival Study Group. Randomized trial of cholesterol
lowering in 4444 patients with coronary heart disease: the Scandinavian
Survival Study. Lancet 1994;344:1383. (The
first major, randomized, prospective study of statin efficacy in
patients with CHD; shows a 42% reduction in CHD mortality and a 30%
reduction in all-cause mortality with no increased risk of noncardiac
death.)
42. Grundy
SM, Cleeman JI, Bairey CN, et al. Implications of recent clinical
trials for the National Cholesterol Education Program Adult Treatment
Panel III guidelines. Circulation. 2004;110:227. (Evidence-based
consensus revisions of the guidelines, based on emerging data from
major RCTs of intensive therapy in high-risk patients; suggests
optional lowering of LDL-cholesterol treatment thresholds and goals in
such patients.)
P.206
Appendix
Appendix I
Appendix I. Fatty Acid Composition of Commonly Consumed Foods (as Percentage of Total Fatty Acids) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Appendix II
Appendix II. Cholesterol Content of Common Foods | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Appendix III
Appendix III. Fat Content of Meats, Poultry, Fish, and Other Protein Sources, 3-Ounce Portions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||