

The purpose of today’s lecture is to discuss the biosynthesis and metabolism of additional major plant carbohydrate groups particularly those involved in cell wall structure and function.
Reading Assignments for the 3rd carbohydrate discussion
a) REQUIRED:
1- Chapter 2, pp 45-110, of the Biochemistry & Molecular Biology of Plants class text.
2- Pauly, M., and Keegstra, K. (2016). Biosynthesis of the Plant Cell Wall Matrix Polysaccharide Xyloglucan. In Annual Review of Plant Biology, Vol 67, S.S. Merchant, ed., pp. 235-259.
b) OPTIONAL:
1- Carroll, A., and C. Somerville. 2009. Cellulosic Biofuels. Annual Review of Plant Biology 60:165-182.
2- Harris, D., V. Bulone, S.-Y. Ding, and S. DeBolt. 2010. Tools for cellulose analysis in plant cell walls. Plant Physiol.:pp.110.154203.
3- Vandavasi, V.G., Putnam, D.K., Zhang, Q., Petridis, L., Heller, W.T., Nixon, B.T., Haigler, C.H., Kalluri, U., Coates, L., Langan, P., et al. (2016). A Structural Study of CESA1 Catalytic Domain of Arabidopsis Cellulose Synthesis Complex: Evidence for CESA Trimers. Plant Physiol 170, 123-135.
4- Himmel, M.E., S.-Y. Ding, D.K. Johnson, W.S. Adney, M.R. Nimlos, J.W. Brady, and T.D. Foust. 2007. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 315:804-807.
5- Atmodjo, M. A., Hao, Z., Mohnen, D. 2013. Evolving Views of Pectin Biosynthesis. Annual Review of Plant Biology. 64:747-779.
6- Bosca, S., C.J. Barton, N.G. Taylor, P. Ryden, L. Neumetzler, M. Pauly, K. Roberts, and G.J. Seifert. 2006. Interactions between MUR10/CesA7-Dependent Secondary Cellulose Biosynthesis and Primary Cell Wall Structure. Plant Physiol. 142:1353-1363.
7- Mutwil, M., S. Debolt, and S. Persson. 2008. Cellulose synthesis: a complex complex. Current Opinion in Plant Biology 11:252-257.
Cell Wall Polysaccharides
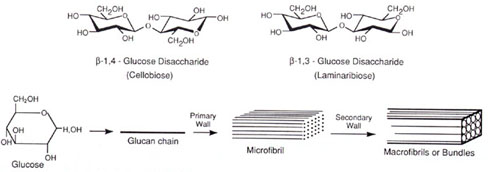
Cellulose is by far the earth's most abundant biological polymer. It is a major structural component of the plant cell wall or extracellular matrix. Like amylose cellulose is a linear homopolymer of D-glucose units with carbon 1 of the non-reducing end linked to carbon 4 of the glucose on the reducing end. However, for cellulose the glycosidic linkages are ß-1,4 rather than α-1,4 in the case of amylose. This seemingly small difference has enormous effects on the structures of these two molecules. Glucose units linked α-1,4 are slightly bent and such polymers tend to adopt helical or corkscrew conformations. Cellulose with ß-1,4 glycosidic bonds can adopt a fully extended conformation with the more stable alternating 180° flips of the glucose units:
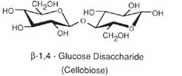
Enzymes which hydrolyze α-1,4 glycosidic linkages have no activity with ß-1,4 glycosidic bonds and likewise ß- glucosidases have no activity with a bonds.
Cellulose is one of the principal components of both primary and secondary plant cell walls being up to 40% of secondary cell walls. The degree of polymerization of cellulose in primary walls is ~2,000-6,000 glucose units and ~10,000 residues in secondary walls. These cellulose polymers are packed parallel to each other in structures known as microfibrils composed of ~36 cellulose chains. In secondary walls these microfibrils are often further associated in bundles or macrofibrils. See Delmer and Amor (1995) The Plant Cell 7, 987-1000, Gardner et al. (2003) and Himmel et al. (2007):
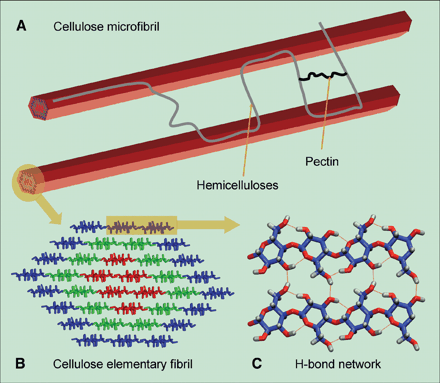
This allows intra-chain, inter-chain and inter-sheet hydrogen bonding in the cellulose fibrils resulting in a structure of great strength:
![[Image of cellulose fibers]](fig10-27.jpg)
Cellulose is synthesized in a multi-subunit complex in the plasma membrane with each unit of the complex responsible for polymerization, secretion, alignment and possibly crystallization of each cellulose chain of the microfibrils. Sucrose synthase provides the glucose needed for cellulose synthesis in the form of UDP-glucose and cellulose synthase catalyzes the ß-1,4 glycosidic bond formation of the cellulose polymers. This complex with ~36 units (for each cellulose polymer of a microfibril) is hypothesized to move in the plasma membranes and the pattern of movement is guided by microtubules that are adjacent or connected to the synthase complex. See Fig. 2 of Delmer and Amor (1995) The Plant Cell 7, 987-1000:
![[Image of complex]](cellfig2.jpg)
Ding and Himmel (2006) present a model (Fig. 3) based on direct visualization of maize stem pith parenchyma cell walls using high-resolution atomic force microscopy. They propose that the cellulose synthases form honeycomb arrayed rosettes directing formation of both microfibrils and macrofibrils:
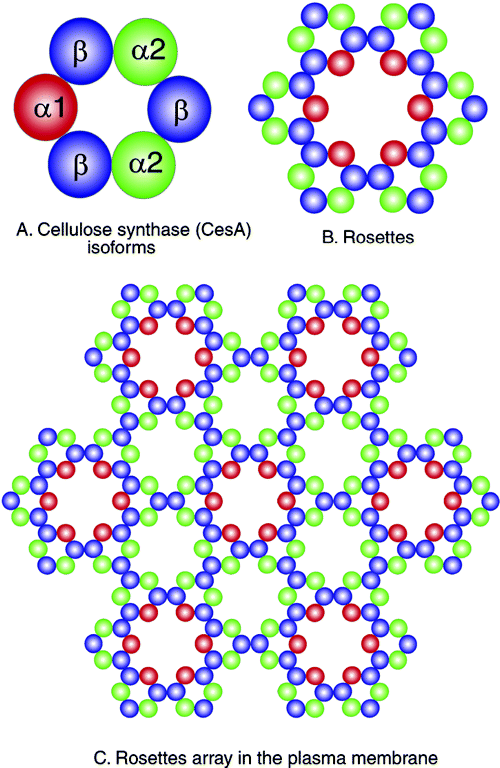
Vandavasi et al. (2016) report cellulose synthase trimers in groups of 6 makint 18-chain microfibrils in Arabidopsis (see Fig. 10).
As cells grow the macrofibril can split into microfibrils and become coated with hemicelluloses forming a stiffness to the microfibril network in cell walls Ding and Himmel (2006).
Most all plant cells have primary cell walls. Plant tissues with only primary cell walls are rather soft and their rigidity is maintained by turgor pressure. Wilting of such plant tissues can occur when they do not contain sufficient water to maintain turgor pressure. Some terminally differentiated plant cells have extensive additional cell wall deposits inside the primary walls known as secondary walls that can be several times the thickness of the primary walls. The secondary walls are often laid down in layers with the cellulose microfibrils having different but defined orientations (Dey & Harborne, 1997):
![[Image of wall structure]](fig5-3.jpg)
In addition to cellulose and lignin of secondary walls, plant cell walls contain an amorphous matrix composed of cross-linking glycans, pectic substances, proteins and lipids:
![[Image of amorphous matrix]](firg5-2.jpg)
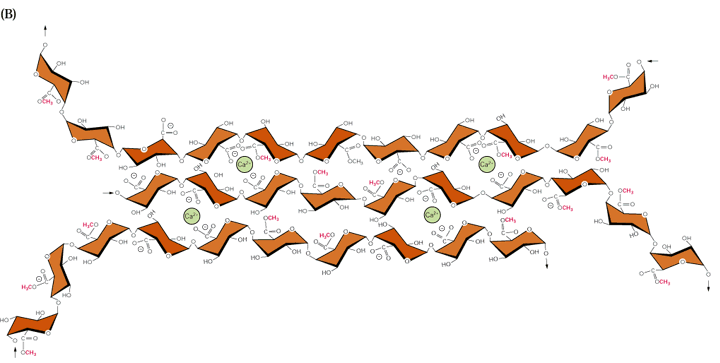
Hemicellulose is a common but archaic term for all substances extractable from plant cell walls with molar concentrations of alkali that can be referred to as cross-linking glycans. These are a diverse group of carbohydrate polymers that vary among different plant groups and between primary and secondary walls. Like cellulose, cross-linking glycans are chains of sugar monomers mostly joined by ß-1,4 glycosidic linkages. However unlike cellulose, the chain lengths are shorter (e.g. several hundred residues), they are composed of various monomer units and the ß-1,4 linked backbone contains numerous short side chains that might be linked α1,2, α1,3 or α1,6. Also some primary cell wall cross-linking glycans backbones contain ß-1,3 in addition to ß-1,4 linkages. The sugar monomers of plant cell walls include glucose, mannose, xylose, arabinose, galactose and 4-O-methyl glucuronic acid. Fig. 2.9 Class text (Fig. 2.10 new text):
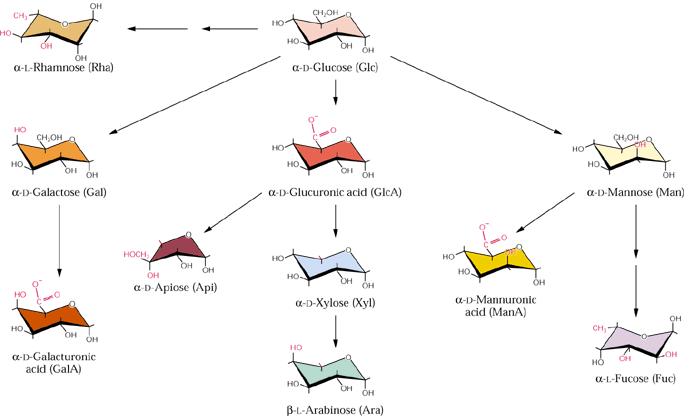
Fig. 2.12
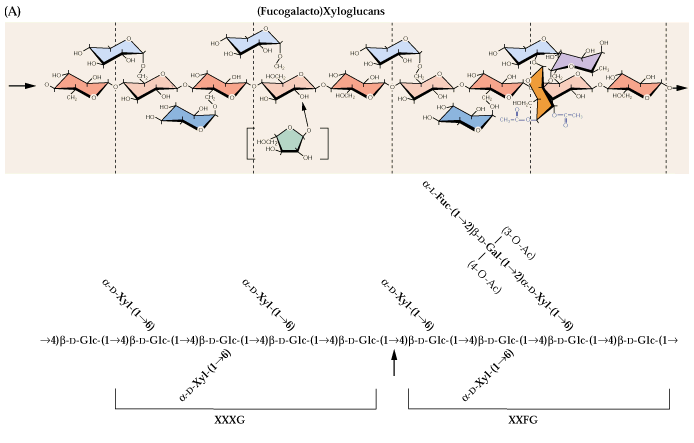
Fig. 2.13
Pectins are another complex group of polysaccharides that are abundant in primary cell walls and in the middle lamella between all plant cells. Like hemicellulose, pectin polymers are chemically diverse molecules. Pectins are acidic and contain a high proportion of D-galacturonic acid residues joined by α-1,4 glycosidic linkages. Some of the carboxylic acids of the galacturonates are esterified to methanol. L-rhamnose (a 6-deoxyhexose) residues are usually interspersed throughout the chain. The linkage of D-galacturonic acid to L-rhamnose is α-1,2 and the linkage from D-galacturonic acid to the next galacturonic acid in the chain is a-1,4. Side chains are often attached to these rhamnose residues. The side chains appear to be neutral sugars polymers containing monomers such as L-arabinose or D-galactose. These types of pectins are known as rhamnogalacturonans. Neutral pectins are also known which appear to be large branches of the acidic pectins. The 3 main types are the arabinans, the arabinogalactans and the galactans. Arabinans are highly branched consisting of a core of α-1,5 arabinosyl (a pentose in the furanose ring form) residues containing α-1,3- and α-1,2-linked arabinosyl side chains. The arabinogalactans contain ß-1,4-linked galactose chains carrying arabinose residues at the 3 and 6 positions that are further substituted. Galactans are mostly linear ß-1,4-linked D-galactose polymers with occasional single L-arabinose branches (Bacic, 2006).
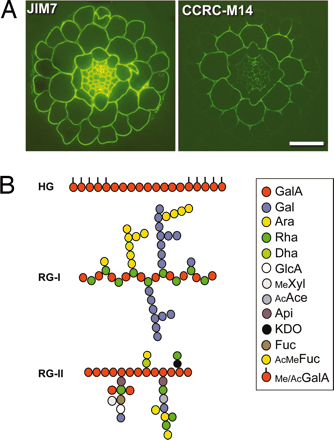
Fig. 2.16 class text:
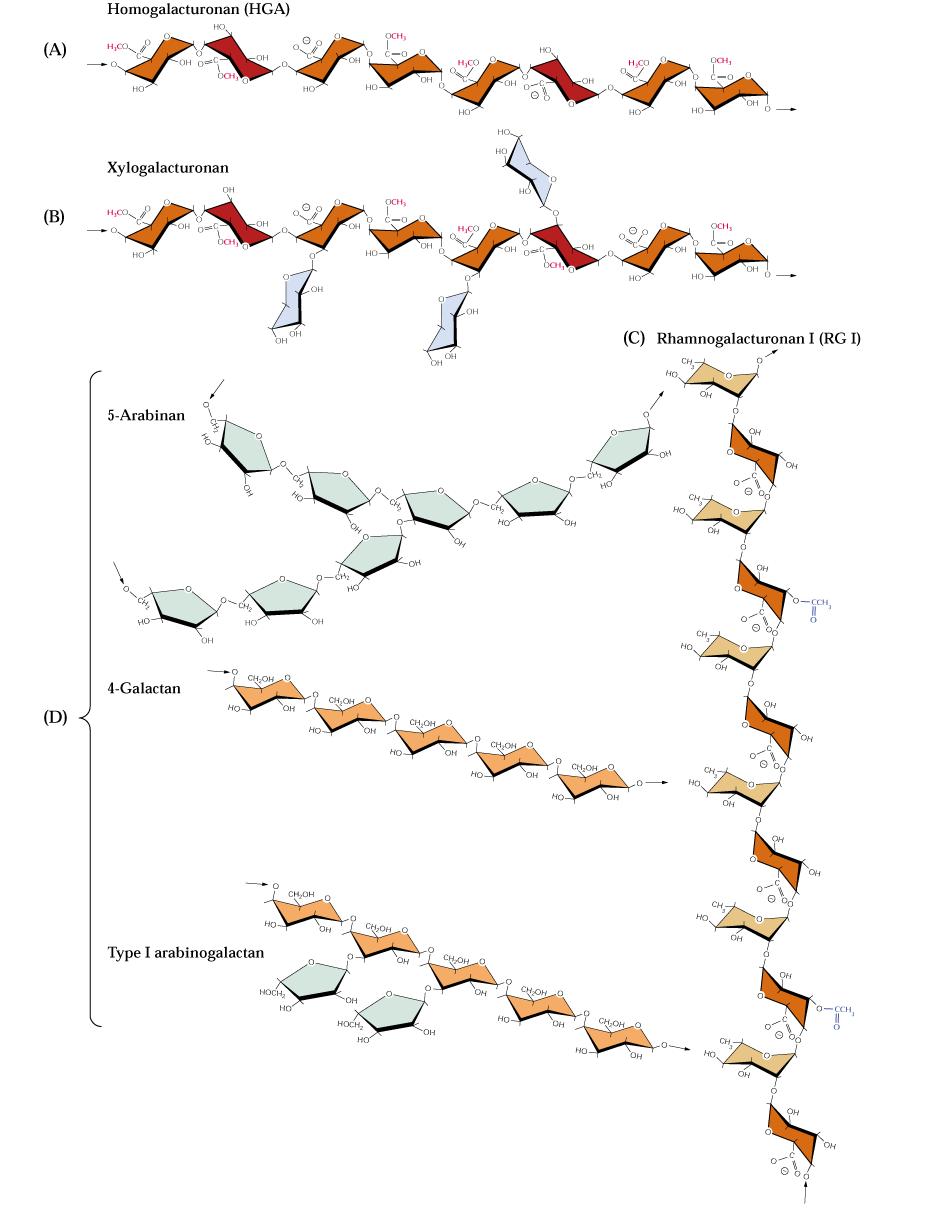
At least some of the xyloglucans of primary cell walls are bound to cellulose. They likely can tether cellulose microfibrils together as illustrated in Dey & Harborne (1997):
![[Image of cellulose microfibril]](fig5-15.jpg)
The first functional model of primary cell walls of plant cells was put forth by Albersheim et al. It suggests a framework of cellulose microfibrils linked to a xyloglucan-pectin-protein matrix. In this model the xyloglucan hydrogen-bonded to cellulose is also hydrogen-bonded to arabinan and galactan side chains of the pectin polymer and the arabinogalactan side chain of pectin is attached to serine of the hydroxyproline rich wall glycoprotein:
![[Image of framework of cellulose microfibrils]](fig5-18.jpg)
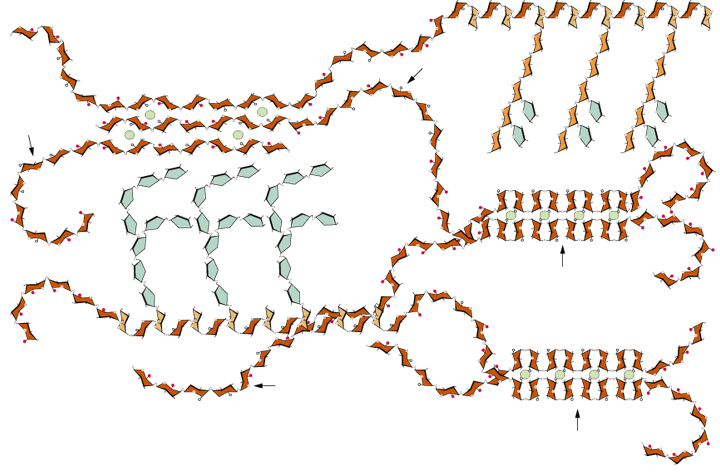
Another model is based on the hypothesis that there are independent polymer networks within the (primary) cell wall; a cellulose-xyloglucan network, a pectin network and a protein network (Carpita & Gibeaut, 1993):
![[Image of polymer networks, part one]](fig5-19.jpg)
![[Image of polymer networks, part two]](fig5-20.jpg)
The spatial arrangement of primary wall polymers is schematically illustrated by McCann & Roberts (1991).
Fig. 2.22 class text:
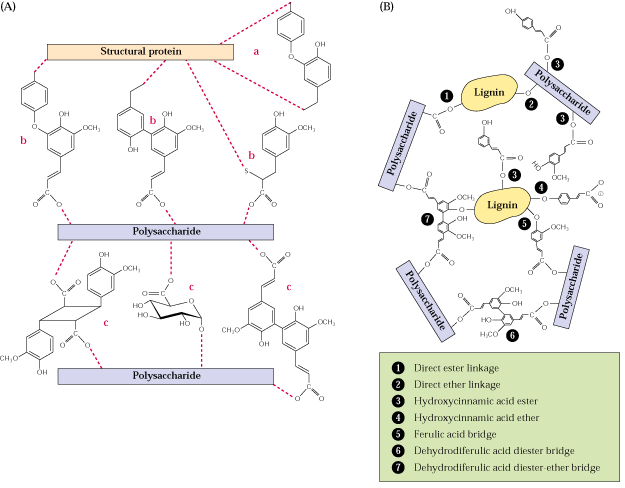
Fig. 2.25:
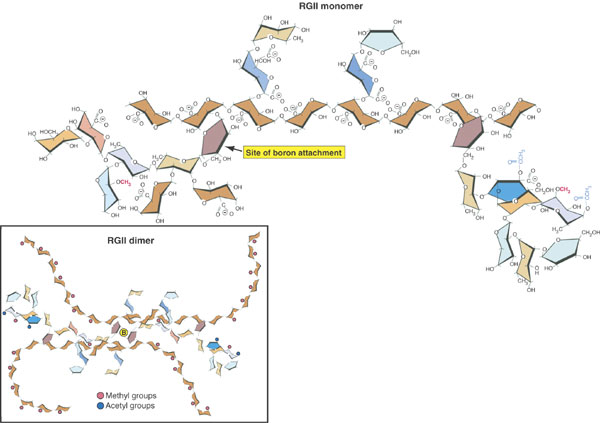
Most pectic polymers are heterogeneous but rhamnogalacturonan II (RGII) is highly conserved among plant species. It is not readily digested even in fermented products so large amounts can accumulate in these products such as wine. RGII monomers form dimers through boron diesters with apiose residues which is necessary for normal plant growth (O'Neill et al., 2001; Hofte, 2001; Fig. 2.17a and b):
We only have time to briefly discuss some other important carbohydrate polymers.
A major structural component in biology is chitin that is very similar to cellulose in primary, secondary and tertiary structure. The only difference between chitin and cellulose is that the C-2 hydroxyl group of the glucose monomers is replaced by NHCOCH3 so that the repeating units are N-acetyl-D-glucosamines (see Jakubowski's online text for a comparison):
Chitin is a fundamental component of fungal cell walls and arthropod exoskeletons. Although higher plants do not generally contain chitin, chitinase is a common plant defense protein.
The ß-1,3 glucan, callose, also similar to cellulose, is an important polymeric component of sieve plates of phloem tubes. Callose is also produced during wound healing of damaged plant tissues [see fig.on ß-1,3 glucose above].
Alginates
Another interesting group of structural carbohydrates that form extended hydrogen-bonded ribbons is the alginates of marine brown algae. These include poly(ß-D-mannuronate) and poly α-L-guluronate) which are ß-1,4 linked chains of ß-D-mannuronic acid and α-L-guluronic acid.
Marine red algae contain the structural polysaccharide agar, which consists of 2 components, agarose and agaropectin. Agarose is composed of alternating D-galactose and 3,6-anhydro-L-galactose with side chains of 6-methyl-D-galactose residues. Agaropectin is like agarose but additionally contains sulfate ester side chains and D-glucuronic acid. The tertiary structure of agarose is a double helix with a so-called threefold screw axis. The central cavity of this double helix can accommodate H2O molecules. Agarose and agaropectin readily form gels that contain high amounts of H2O (up to 99.5%). An agarose double helix:
![[Image of an agarose double helix]](fig10-31.jpg)
Gums
Many of the gums used in food and industrial products are carbohydrate derivatives. For thousands of years Mayans and Aztecs chewed a gum from the dried sap of the Sapodilla tree (Manilkara zapotilla, Sapotaceae) known as "chicti" by the Aztecs. This is called "chicle" in the US and in 1880 was sold as Yucatan gum (later Chicle gum) after combining with corn syrup and peppermint. Gums are polysaccharides containing sugar acid monomers similar to the cell wall polymers hemicelluloses and pectins. They are formed in wounds of many woody plants and appear to be involved in sealing wounds.
Food Applications
One of the most important issues in health and nutrition today is non-digestable dietary fiber. What is meant by this?
Water modifying substances are called hydrogels in food science. The proteins known as gelatin obtained from boiled, hydrolyzed skin, tendons, ligaments, bones, etc. have such properties. From plants starches, pectins, gums, agar derivatives and carrageenan (from the red alga, Chondrus crispus or Irish moss), all carbohydrates, fulfill this purpose. They are used as thickeners and stabilizers in a variety of foods including ice cream, puddings, jellies, confections and many modern prepared food products. Gums are used to coat many "instant mixes" to minimize water adsorption from the atmosphere and therefore reducing clumping of the mixes.
`Non-digestable carbohydrates', especially those soluble in water are currently of great interest in human foods and nutrition. This includes most of the carbohydrates discussed in this lecture except starch and many simple sugars and food processors have been working toward modifying starch to reduce its digestibility.
Are fructans readily digestible?

β-Glucans, oat bran (Fig. 2.14)...chia..., chia health benefits
Fig. 2.43:

Preliminary Reading Assignment for the First Lipids Discussion:
a) REQUIRED:
1- Fich, E.A., Segerson, N.A., and Rose, J.K.C. (2016). The Plant Polyester Cutin: Biosynthesis, Structure, and Biological Roles. In Annual Review of Plant Biology, Vol 67, S.S. Merchant, ed., pp. 207-233.
2- Chapter 8 sect. 8.1 - 8.4, pp 337 -352 & sect. 8.9 pp 382 - 389 (Chapt 10 sect. 10.1 - 10.4, pp 456-476 old text) of the Biochemistry & Molecular Biology of Plants class text.
b) OPTIONAL:
1- Maier, T., M. Leibundgut, and N. Ban. 2008. The Crystal Structure of a Mammalian Fatty Acid Synthase. Science 321:1315-1322.
2- Yeats, T. H. and J. K. C. Rose. 2013. The Formation and Function of Plant Cuticles. Plant Physiology 163: 5-20.
3- Jetter, R., and Riederer, M. (2015). Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiology.
4- Samuels, L., L. Kunst, and R. Jetter. (2008). Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annual Review of Plant Biology 59:683-707.
5- Focks, N. and C. Benning. 1998. wrinkled 1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118:91-101.
| All materials © 2018 David Hildebrand, unless otherwise noted. | |||||||
| home | syllabus | lecture schedule & web notes | virtual office hours | messages & answers from the instructor | supplementary material | related links | What's new? |