THE BIOCHEMICAL BASIS
FOR TOBACCO BUDWORM, HELIOTHIS VIRESCENS, TOLERANCE TO CANAVANINE
INTRODUCTION
As documented previously, the higher plant nonprotein amino acid
L-canavanine is a potent arginine antimetabolite that
is typically insecticidal to non-adapted insects (Rosenthal, 1982; Rosenthal
and Bell, 1979). For example, terminal instar larvae of the tobacco hornworm,
Manduca sexta [Sphingidae], reared on an agar-based diet supplemented
with 2.5 mM canavanine, produced pupae and adults that exhibited striking
developmental aberrations.
(pictures here(
In marked contrast, larvae of the tobacco budworm,
Heliothis virescens [Noctuidae], a highly destructive agricultural
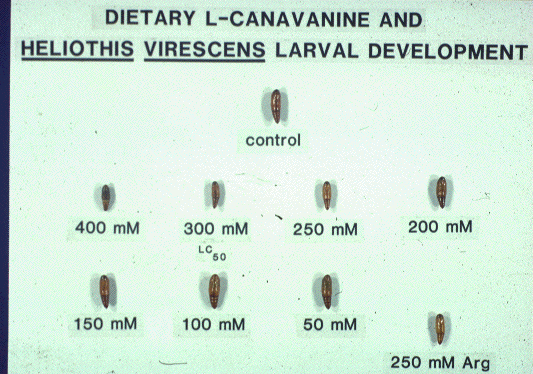
pest (Lincoln, 1972) reared on canavanine-containing diet had an LC50
value for this arginine antagonist of 300 mM (Berge et al., 1986). 
This LC50 corresponds to 53,000 ppm wet diet weight or nearly
40% on a dry weight basis (Berge et al., 1986). While the pupae that emerged
from these canavanine-treated larvae were depauperate, they lacked discernible
developmental aberrations. A group of five larvae sustained on a staggering
500 mM canavanine-containing diet survived for 9 days before the first
larval dead was recorded.
Heliothis virescens resistance to this arginine antagonist was
also demonstrated by parenteral injection of canavanine: the LD50
for this nonprotein amino acid in M. sexta was 1.0 g kg-1 fresh
body weight as compared to 10.7 g kg-1 for H. virescens (Berge et
al., 1986). Heliothis virescens might tolerate elevated levels of
dietary canavanine because of its ability to excrete this potentially toxic
allelochemical. Analysis of the larval fecal matter, produced by terminal
instar larvae from feeding on 150 mM canavanine-containing diet, disclosed
that only 0.6% of the consumed canavanine was eliminated by this excretory
route. Examination of the body fluids and tissues established the lack
of significant sequestration of canavanine within the larval body (Berge
and Rosenthal, 1990). Thus, H. virescens actively metabolized canavanine;
the t1/2 for canavanine clearance from
the hemolymph was 135 min (Berge and Rosenthal, 1990).
LARVAL ABILITY TO TOLERATE CANAVANINE
Biochemical studies were conducted to determine if larval ability to
tolerate canavanine resulted from its constitutive metabolism, or alternatively
was induced in response to dietary consumption of canavanine (Berge and
Rosenthal, 1990). In one study, the t1/2 for
canavanine clearance of parenterally injected L-[guanidinooxy-14C]canavanine,
in larvae maintained on a 150 mM canavanine-containing diet for 72 h, was
138 min. This is exactly the same value found for control insects that
were not exposed previously to canavanine prior to determining the t1/2
value for canavanine clearance.
In another approach to resolving this question, H. virescens
larvae were provided sufficient cycloheximide to inhibit nearly 80% of
the L-[3H]leucine-labeled protein formation
observed in control animals. Such cycloheximide-treated insects cleared
canavanine with a t1/2 equal to 121 min;
once again this value was identical in control animals (Berge and Rosenthal,
1990). Electrophoretic analysis of hemolymph proteins of larvae reared
on 150 mM canavanine-containing diet was identical to that obtained from
larvae raised on control diet.
IN SUMMARY, NONE OF THE DESCRIBED EXPERIMENTS
PROVIDED EVIDENCE FOR SYNTHESIS OF A UNIQUE PROTEIN BY CANAVANINE-TREATED
INSECTS. THIS RESULT SUPPORTED THE VIEW THAT H. VIRESCENS DREW UPON
A PREEXISTING, CONSTITUTIVE ENZYME TO CATABOLIZE CANAVANINE.
Analysis of the various body organs of the larvae demonstrated that
canavanine catabolism occurred solely in the gut. In particular, the fat
body, the rough insectan equivalent of the mammalian liver and a major
detoxification organ, did not possess significant canavanine-degrading
ability. Moreover,
the canavanine-catabolizing enzyme of the larvae was found exclusively
in the gut, and was probably part of the gut wall rather than secreted
into the gut lumen since this enzyme was not lost when the gut contents
were removed by thorough washing.
the microbody fraction of an extract of the larval gut could not
degrade canavanine; this ability resided solely in the soluble fraction
of the gut extract.
CANAVANINE CATABOLISM
To unravel the metabolic disposition of canavanine, the larvae were
provided L-[guanidinooxy-14C]canavanine.
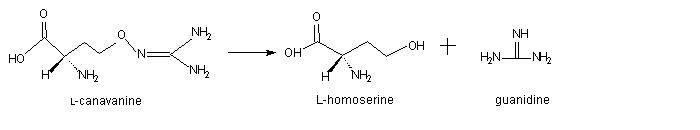
The principal degradation product of radiolabeled canavanine was [14C]guanidine(Berge
and Rosenthal, 1991). Subsequent in vivo studies employing L-[1,2,3,4-14C]canavanine
identified L-[1,2,3,4-14C]homoserine as
the preponderant radiolabeled catabolite. This finding immediately suggested
the following pathway in which the 14carbon atom of the guanidinooxy
moiety of canavanine was transferred to guanidine:
Other experiments with larvae of H. virescens led to the discovery
of a gut enzyme--a larval reductase able to catalyze an NADH-dependent
reduction of hydroxyguanidine to guanidine (reference). This finding created
the intriguing possibility that canavanine was catabolized initially to
homoserine and hydroxyguanidine rather than guanidine by a novel hydrolase
able to cleave the O-N bond of the guanidinooxy moiety of the substrate.
 While
This metabolic ability has been observed in a soil-borne Pseudomonas
(reference), the responsible enzyme was not isolated nor has this metabolic
capacity been described from an eukaryotic organism.
While
This metabolic ability has been observed in a soil-borne Pseudomonas
(reference), the responsible enzyme was not isolated nor has this metabolic
capacity been described from an eukaryotic organism.
THE EXPERIMENTAL EVIDENCE INDICATED TWO ENZYMES
WERE FUNCTIONING IN CONCERT TO METABOLIZE CANAVANINE. THE FIRST REACTION
EMPLOYED A NOVEL HYDROLASE THAT DIRECTED THE FORMATION OF HOMOSERINE AND
HYDROXYGUANIDINE FROM CANAVANIE. IN THE SECOND REACTION, HYDROXYGUANIDINE
WAS REDUCED TO GUANIDINE.
CANAVANINE HYDROLASE
The existence of canavanine hydrolase (CH), an
enzyme able to cleave an oxygen-nitrogen bond, would be an important finding
since this enzyme is the only protein known to demonstrate this ability.
As such it would represents a new type of hydrolase-- one that acts on
oxygen-nitrogen bonds (EC 3.13.1.1).
The search for this enzyme in the gut of larval H. virescens
culminated in the isolation of a homogeneous enzyme that mediated an irreversible
hydrolysis of L-canavanine to L-homoserine and hydroxyguanidine. Canavanine
hydrolase (EC 3.13.1.1) exhibited a high affinity for canavanine as judged
by the apparent Km value of 1.1 mM for canavanine. The turnover
number for this reaction was 21.1 mmol min-1 mmol-1.
Canavanine hydrolase also exhibited a high degree of specificity for L-canavanine
as it could not function effectively with either L-2-amino-5-(guanidinooxy)pentanoate
or L-2-amino-3-(guanidinooxy)propionate, the higher or lower homolog, respectively
of L-canavanine nor its methyl ester. Canavanine derivatives such as L-canaline
and O-ureido-L-homoserine were not metabolized significantly by canavanine
hydrolase.
Literature cited
Bell, E. A., Lackey, J. A. and Polhill, R. M. (1978) Systematic significance
of canavanine in the Papilionoideae (Faboideae). Biochem. Syst. Ecol. 6,
201-212.
Berge, M.A. and Rosenthal, G.A. (1990) Detoxification of L-canavanine
by the tobacco budworm, Heliothis virescens [Noctuidae]. J. Food Agr. Chem.
38, 2061-2065.
Berge, M. A. and Rosenthal, G. A. (1991) Metabolism of L-canavanine
and L-canaline in the tobacco budworm, Heliothis virescens [Noctuidae].
Chem. Res. Toxicology 4, 237-240.
Berge, M. A., Rosenthal, G. A. and Dahlman, D. L. (1986) Tobacco budworm,
Heliothis virescens [Noctuidae] resistance to L-canavanine, a protective
allelochemical. Pestic. Biochem. Physiol. 25, 319-326.
Brazzel, J. R., Newsom, L. D., Roussel, J. S., Lincoln, C. and Williams,
F. J. (1953) Bollworm and tobacco budworm as cotton pests in Louisiana
and Arkansas. La. Agric. Exp. Stn. Tech. Bull. 482, 47 pp. Fitt, G. P.
(1989) The ecology of Heliothis species in relation to agroecosystems.
Ann. Rev. Entomol. 34, 17-52.
Lincoln, C. (1972) Distribution, abundance and control of Heliothis
spp. in cotton and other host plants., Oklahoma Agricultural Experimental
Station, Oklahoma State University, Stillwater, OK.
Natelson, S. (1985) Canavanine to arginine ratio in alfalfa (Medicago
sativa), clover (Trifolium), and the Jack bean (Canavalia ensiformis).J.
Agric. Food Chem. 33, 414-419.
Pearson, E.O. (1958) The Insect Pests of Cotton in Tropical Africa.
Commonw. Inst. Entomol., London, UK.
Rosenthal, G. A. (1977) The biological effects and mode of action of
L-canavanine, a structural analogue of L-arginine. Q. Rev. Biol. 52, 155-178.
Rosenthal, G.A. (1982) Plant Nonprotein Amino and Imino Acids: Biological,
Biochemical, and Toxicological Properties. 273 pp. Academic Press, San
Diego, CA.
Rosenthal, G. A. (1992) Radiochemical synthesis and colorimetric analysis
of hydroxyguanidine. Bioorganic Chem. 20, 55-61.
Rosenthal, G.A., and Bell, E.A. (1979) In: Herbivores: Their Interaction
with Secondary Plant Metabolites (Rosenthal, G.A., and Janzen, D.H. eds.)
pp. 353-385, Academic Press, San Diego, CA.
Zalucki, M. P., Daglish, G., Firempong, S. and Twine, P. (1986) The
biology and ecology of Heliothis armigera (Hubner) and H. punctigera Wallengren
(Lepidoptera: Noctuidae) in Australia: What do we know? Aust. J. Zool.
34, 779-814.




 While
This metabolic ability has been observed in a soil-borne Pseudomonas
(reference), the responsible enzyme was not isolated nor has this metabolic
capacity been described from an eukaryotic organism.
While
This metabolic ability has been observed in a soil-borne Pseudomonas
(reference), the responsible enzyme was not isolated nor has this metabolic
capacity been described from an eukaryotic organism.