 JESS
SCHWARTZ JEWISH COMMUNITY
JESS
SCHWARTZ JEWISH COMMUNITY
HIGH SCHOOL
4645 E. Marilyn Rd.
Phoenix, Arizona 85032
 CHEMISTRY
WEB SITE
CHEMISTRY
WEB SITE
topic 004
The International
System of Units
The International System of Units or Systeme Internationale (SI)
is an improved metric system adopted by the Eleventh General Conference
of Weights and Measures in 1960. It is the universal measuring system used
in all areas of science throughout the world. The entire SI system of measurement
is constructed from seven base units, each of which represents a
single physical quantity as shown in the table below. For our chemistry
class, we shall only consider five of these units.
Base Units of the International System
Quantity Name of Unit Unit Symbol
length meter (metre) m
mass kilogram kg
time second s
temperature kelvin K (case sensitive)
amount of substance mole mol
Like earlier versions of the metric system, the SI units can be designated
as decimal fractions or multiples by the use of appropriate prefixes. The
acceptable SI prefixes are given in the table below.
Prefixes of the International System
Factor Prefix Symbol Factor Prefix Symbol
10e-15 femto f
10e12 tera T 10e-12 pico p
10e9 giga G 10e-9 nano n
10e6 mega M 10e-6 micro(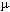 )
10e3 kilo k 10e-3 milli m
10e-2 centi c
)
10e3 kilo k 10e-3 milli m
10e-2 centi c
These prefixes are of critical importance,
you must take the time to become completelyl familiar and comfortable with
them!
N.B. Whenever exponents are used with SI prefixes on either
base units or derived units, the exponent applies to the prefix as well
as to the unit. For example, nm2, or square nanometer, is interpreted
as (nm)2 rather than n(m2). Any prefix can be applied
to any base unit except the kilogram; the kilogram takes prefixes
as if the base unit were the gram. As a consequence 10-6 kg
is written as 1 milligram (mg) rather than 1 microkilogram.
The great advantage of the SI over other systems of units is that when
any physical quantity whatever is written out in the SI base units or in
units derived only from the SI base units, any mathematical manipulations
performed with them will follow the quantity calculus. No conversion
factors will ever be required. This means that if the symbols in any
equation are replaced by real numbers with their SI base units and algebraic
manipulations are performed upon the units in exactly the same way as they
are performed upon the numbers to which those units refer, the result will
come out with the correct numbers and units.
Example. The mass of a sample of pure rhombic sulfur was 150.637 g and
the volume of water it displaced was 72.8 mL. The density of sulfur is
then (150.637 g)/(72.8 mL) = 2.07 g/mL, or g/cm3, or kg/dm3,
or kg/L.
Base Units of the SI
LENGTH
The SI unit of length is the meter, a fundamental
unit of the SI. The meter was once defined in terms of the circumference
of the earth as part of the older metric system. Since 1983 the meter is
by definition the length of the path travelled by light in vacuum in 1/299,792,458
of a second. ,
Some units of length worth memorizing: one inch =25.4 mm exactly,
meter, centimeter, millimeter, micrometer (micron), nanometer and the Angstrom
(derived unit).
You need to be familiar with the km, m, cm, mm, 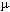 m
or micron, and a nanometer. Also, 10 nm are equal to 1 angstrom (A).
m
or micron, and a nanometer. Also, 10 nm are equal to 1 angstrom (A).
MASS
The SI unit of mass is the kilogram, a fundamental
unit of the SI. The kilogram was once
defined as the mass of one cubic decimetre (L) of water. Since 1901 it
is by definition the mass of the international prototype of the kilogram,
a platinum-iridium mass which is stored at Sevres in France.
An interesting fact about the kilogram is that it is the only SI base
unit to incorporate a prefix. The kilogram is the only SI unit based on
a finite amount of material rather than some physical parameter.
You need to be familar with the kg, g, mg, 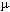 g
and ng
g
and ng
TIME
The SI unit of time is the second, a fundamental
unit of the SI. Originally defined in terms of the rotation of the
earth, the second is now defined in terms of atomic transitions in 133Cesium
because these are subject to more precise measurement. Specifically, since
1967 the second is defined as the duration of 9,192,631,770 periods of
the electromagnetic radiation corresponding to the transition between the
two hyperfine levels of the ground state of the 133Cs atom.
TEMPERATURE
The SI unit of temperature is the kelvin, a fundamental
unit of the SI. Since 1967, the kelvin has been by definition the
fraction 1/273.16 of the thermodynamic temperature of the triple point
of water. The triple point of water is the temperature at which ice, water,
and water vapor can all exist in equilibrium and its value is +0.01o
Celsius.
The kelvin (which is correctly written without a degree sign) is used
for measuring both temperature and temperature interval; thus one can say,
"The temperature is 300 K" or "This pan is 20 K hotter than that one."
Temperatures in kelvin can only be positive and so they require no sign.
The kelvin scale of temperature is also known as the absolute scale and
the thermodynamic scale. Conversion factors between temperatures in degrees
Celsius (oC) and temperatures in kelvin are:
|
Temperature (oC) + 273.15 (exactly)
= temperature (K) (memorize this)
|
N.B. The degree Celsius, the unit of the common metric temperature scale,
is not part of the SI but its use is not discouraged. A temperature interval
in degrees Celsius is identical to a temperature interval in kelvin, although
a temperature in degrees Celsius is not identical to a temperature in kelvin.
AMOUNT OF SUBSTANCE
The SI unit of quantity or amount of substance is
the mole, a fundamental unit of the SI. There are no other modern
units in which amount of substance is measured, so no conversion factors
are required. Often, however, units of mass or volume are used to give
the amount of substance. Conversion of these to the mole requires the use
of appropriate measured physical constants, the molar mass or the molar
volume. Since 1971, by definition one mole of entities is the same number
of entities as there are atoms of carbon12 in exactly 0.012
kilogram of carbon-12, which is Avogadro's number of entities (approximately
6.0221 x 1023 entities).
 You need to be familiar with
a mol, mmol,
You need to be familiar with
a mol, mmol, 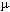 mol,
nmol and a pmol.
mol,
nmol and a pmol.
Non-SI Units Commonly Used (YOU
NEED TO MEMORIZE THESE)
1) Liter: symbol = L.
2) cubic centimeter: symbol = cm3. Often used for measuring
the volume of solids, one cm3 equals
one milliliter (mL). The mL has emerged as the common measure of volume.
3) Ångström: symbol = Å. One Å equals 10¯8
cm. THERE ARE 10Å IN ONE NANOMETER.
 JESS
SCHWARTZ JEWISH COMMUNITY
JESS
SCHWARTZ JEWISH COMMUNITY
 JESS
SCHWARTZ JEWISH COMMUNITY
JESS
SCHWARTZ JEWISH COMMUNITY
 CHEMISTRY
WEB SITE
CHEMISTRY
WEB SITE
) 10e3 kilo k 10e-3 milli m 10e-2 centi c
![]() m
or micron, and a nanometer. Also, 10 nm are equal to 1 angstrom (A).
m
or micron, and a nanometer. Also, 10 nm are equal to 1 angstrom (A).
![]() g
and ng
g
and ng