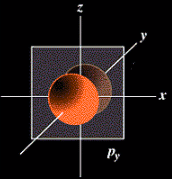
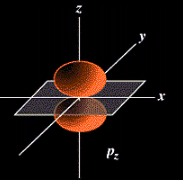
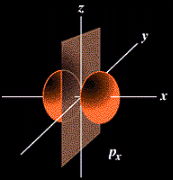
The probable location for the six "p" orbital electrons when all of the p subshell is filled.
Think of these orbitals as being large in volume as you move into higher principal quantum numbers or shells.




The probable location for the six "p" orbital electrons when all of
the p subshell is filled.
Think of these orbitals as being large in volume as you move into higher
principal quantum numbers or shells.
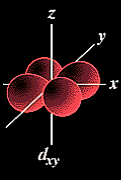



The probable location for eight of the ten "d" orbital electrons when
all of the d subshell is filled.