| The Biomedical Optics Lab develops optical spectroscopy/imaging for quantification of blood flow, oxygenation and oxygen metabolism in normal diseased tissues of animals and humans. The motivation is to explore the feasibility of optical-electronic instruments for noninvasive diagnosis of various diseases and longitudinal monitoring of therapies. The goal is to create a strong biomedical optics research and teaching program, which vertically integrates optics, electronics, engineering and clinical applications. Research interests include: | ||
| ● | Development of Biomedical Instruments | |
| - | Diffuse optical spectroscopy/imaging | |
| - | Multimodality Instrumentation | |
| - | Fiber-optic probe design | |
| ● | Diagnosis of Tissue Disease with Light | |
| - | Cancers (e.g. head/neck, breast, prostate) | |
| - | Peripheral artery disease (PAD) | |
| - | Fibromyalgia disease | |
| - | Stroke | |
| - | Obstructive sleep apnea (OSA) | |
| - | Brain/spinal cord injury | |
| - | Diabetes | |
| ● | Assessment of Treatment Effect | |
| - | Radiation therapy | |
| - | Chemotherapy | |
| - | Surgical revascularization | |
| - | Photodynamic therapy (PDT) | |
| - | Medication | |
|
|
||
| Collaborators |
| - Sibu P. Saha, MD. Prof. of Surgery department of Cardiothoracic Surgery |
| - Mahesh Kudrimoti, Associate Prof., Director Residency Program Radiation Medicine |
| - Karin R. Swartz, Assistant Prof. of Neurosurgery |
| - Sara Selles, Assistant Prof. of Rehabilitation Medicine |
| - Don Hayes, Assistant Prof. of Pediatrics-Pulmonology |
| - David Randall, Prof. of Physiology |
| - Charlotte Peterson, Prof. and Associate Dean of College of Health Science |
| - Leslie J. Crofford, Prof. of Internal Medicine Rheumatology |
| - Brock Symons, Assistant Prof., Graduate Center for Gerontology |
| - Lei Chen, MD, PhD and Michal D. Todorek, MD, PhD in molecular Neurosciences and vascular biology lab, Department of Surgery |
|
|
| Featured Studies | |
| - | Portable optical tissue flow oximeter based on diffuse correlation spectroscopy |
| Brief instruction | |
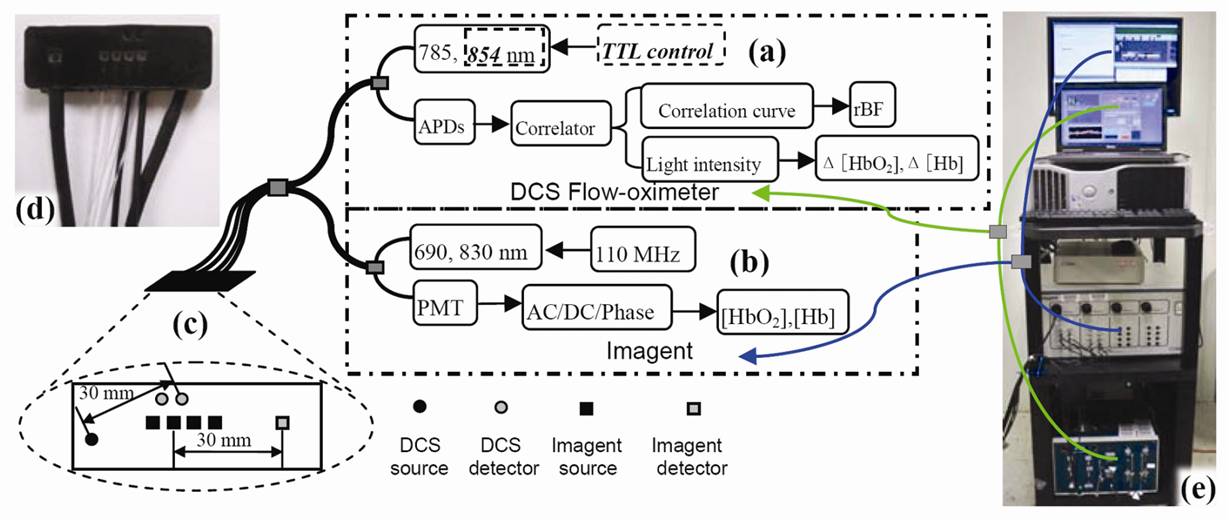
| A portable diffuse correlation spectroscopy (DCS) flowmeter has been extended to measure both tissue blood flow and oxygenation (namely, DCS flow oximeter). For validation purposes, calf muscle blood oxygenation during cuff inflation and deflation was measured concurrently using the DCS flow oximeter and a commercial tissue oximeter (Imagent, ISS Inc.). The oxygenation traces from the two measurements exhibited similar dynamic responses, and data were highly correlated (r(mean)>0.9, P<10(-5), n=10). The portable, inexpensive, and easy-to-use DCS flow oximeter holds promise for bedside monitoring of tissue blood flow and oxygenation in clinics. | |
|
|
|
| Figure: Hybrid system combining the DCS flow oximeter and Imagent: (a) DCS flow oximeter, (b) Imagent, (c) hybrid (d) probe photograph, (e) hybrid system photograph | |
|
View
related
publications [1] Portable optical tissue flow oximeter based on diffuse correlation spectroscopy |
|
| - | Cerebral monitoring during carotid endarterectomy using near-infrared diffuse optical spectroscopies and electroencephalogram |
| Brief instruction | |
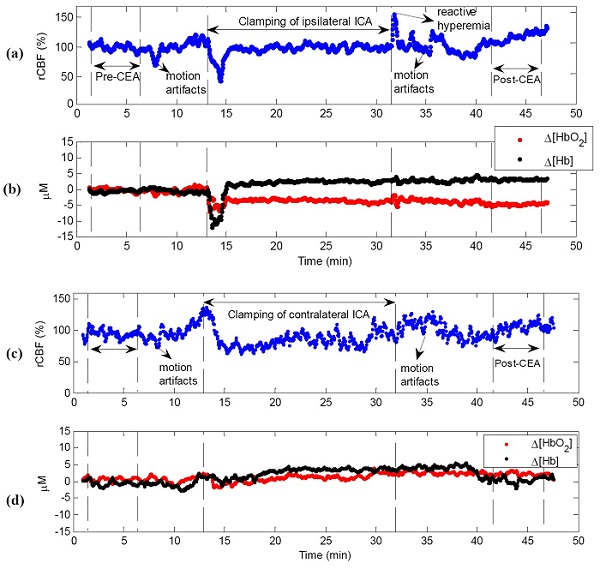
| Intraoperative monitoring of cerebral hemodynamics during carotid endarterectomy (CEA) provides essential information for detecting cerebral hypoperfusion induced by temporary internal carotid artery (ICA) clamping and post-CEA hyperperfusion syndrome. This study tests the feasibility and sensitivity of a novel dual-wavelength near-infrared diffuse correlation spectroscopy technique in detecting cerebral blood flow (CBF) and cerebral oxygenation in patients undergoing CEA. Two fiber-optic probes were taped on both sides of the forehead for cerebral hemodynamic measurements, and the instantaneous decreases in CBF and electroencephalogram (EEG) alpha-band power during ICA clamping were compared to test the measurement sensitivities of the two techniques. The ICA clamps resulted in significant CBF decreases (-24.7 +/- 7.3%) accompanied with cerebral deoxygenation at the surgical sides (n = 12). The post-CEA CBF were significantly higher (+43.2 +/- 16.9%) than the pre-CEA CBF. The CBF responses to ICA clamping were significantly faster, larger and more sensitive than EEG responses. Simultaneous monitoring of CBF, cerebral oxygenation and EEG power provides a comprehensive evaluation of cerebral physiological status, thus showing potential for the adoption of acute interventions (e.g., shunting, medications) during CEA to reduce the risks of severe cerebral ischemia and cerebral hyperperfusion syndrome. | |
 |
|
|
Figure Typical cerebral hemodynamic changes during carotid endarterectomy (CEA). (a) relative cerebral blood flow (rCBF) and (b) cerebral oxygenation changes (Δ[HbO2], Δ[Hb]) at the surgical side; (c) rCBF and (d) Δ[HbO2] and Δ[Hb] at the control side. The vertical dashed lines indicate the beginning and ending of ICA clamping or the periods for calculation of the pre-CEA baseline (5 minutes) and post-CEA value (5 minutes). Note that motion artifacts induced by the surgical procedure (e.g., incision, touching vessels, patient position adjustment) may introduce noises to optical measurements. These motion artifacts are marked and corresponding data are excluded in the data analysis. |
|
| View related publications | |
|
[1]
Portable optical
tissue flow
oximeter based
on diffuse
correlation
spectroscopy [2] Cerebral monitoring during carotid endarterectomy using near-infrared diffuse optical spectroscopies and electroencephalogram |
|