Kentucky Pest News Newsletter
Kentucky Pest News: November 9, 1998
HIGHLIGHTS IN THIS ISSUE
Number 832...........November 9, 1998
ANNOUNCEMENTS
STORED GRAIN
TOBACCO
VEGETABLES
HOUSEHOLD
DIAGNOSTIC LAB HIGHLIGHTS
PESTICIDE NEWS AND VIEWS
PESTICIDE TRAINING MEETINGS
ANNOUNCEMENTS
YEAR-END COMMERCIAL PESTICIDE TRAINING OPPORTUNITIES
The meetings below are convenient opportunities to
receive continuing education credit before
December 31, 1998. Note: All meeting schedules are
listed in local time.
Commercial applicators need to attend 2 approved
training meetings in 5 years to remain certified.
Any questions about the number of meetings
needed by an individual should be directed to the
Division of Pesticides (502) 564-7274.
November 9 - 11-Lexington Convention Center,
Kentucky Turf Conference and Trade Show.
Category 3. Recertification and testing Contact
Mark Wilson (502) 245-1714.
November 19 Holiday Inn North- Lexington,
Pesticide Recertification Training Course- categories
3 (ornamental and turf), 6 (right- of- way), 10 (demo
and research). 1 pm - 4:15 pm. Certification testing
at 4:30. Contact Jim Mathews (502) 627-3206.
November 24 - UK Research and Education Center,
Princeton. Same agenda and times as November 4
meeting.
December 3- 1998 Professional Horticulture
Seminar (Category 3 and 10), IU Southeast Campus,
New Albany, IN 9 am - 4 pm. (Approval pending).
Contact- Roy Ballard, Floyd Co (IN) CES (812) 948-
5470. No certification testing available.
December 15 and 16- Kentuckiana Fertilizer and Ag
Chemical Meeting, Radisson Hotel, Evansville, IN
Categories 1, 10, and 12,
STORED GRAIN
CHECK YOUR GRAIN BINS FOR MOISTURE AND INSECTS
By Doug Johnson
Until recently, it has been a warm dry fall. Large
changes in temperature and humidity can produce
several problems inside grain bins. Combine this
with the large on-farm holdings for the 98 season,
and you have a situation that deserves considerable
attention.
In general, you should change grain temperature
in keeping with that of the outside air. AEN-20, an
Agricultural Engineering publication, provides
information on how to do this over the course of the
year. Obviously, this is important in relation to
grain storage but it also impacts insect infestations.
In turn, insect infestations can keep you from
properly regulating grain temperature and
humidity. Take control of the situation before a
problem occurs and keep your eye open for
problems as the season progresses.
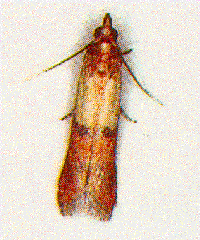
Probably the most common insect problem at this
time of year is the Indian meal moth (IMM).
 Often,
the most common storage problem is sprouted
grain, especially when rainfall follows a long dry
spell. Detecting both of these problems can be
relatively simple. Open the top bin hatch and look
and SMELL inside. Be sure to do this before even
considering entering the bins. (Which by the way
can be very dangerous. Make sure you know what
you are doing. Cut the power to the bins and have a
partner BEFORE entering a bin). A foul or musty
odor is a very good tip that something is wrong.
Often,
the most common storage problem is sprouted
grain, especially when rainfall follows a long dry
spell. Detecting both of these problems can be
relatively simple. Open the top bin hatch and look
and SMELL inside. Be sure to do this before even
considering entering the bins. (Which by the way
can be very dangerous. Make sure you know what
you are doing. Cut the power to the bins and have a
partner BEFORE entering a bin). A foul or musty
odor is a very good tip that something is wrong.
Sprouted grain is usually pretty obvious. IMM also
may be obvious but if the infestation is in an early
stage, you may not notice it. The larvae prefer to
feed on fines or damaged kernels. Infestations are
most common in the upper four to six inches of
grain in a bin. These insects become active in early
spring because surface grain is usually the first to
warm. The larvae produce silken threads which
result in "caking" or "crusting" of the surface grain.
Their frass (waste), cast exoskeletons (exterior
skin-like covering) and silk contaminate the grain. If
this is the case, then look for the presence of larvae
crawling among the mess. The larvae are the
feeding stage and are caterpillars that may
range from yellow white to pink to light green with
a light brown head. Full grown larvae are about 0.7
inch long.
However, you may first notice small moths flying in
and around the grain bins. Indianmeal moths at rest
with wings folded over their backs are about 0.4
inch long. The wingspan is about 0.6 inch. The outer
portion of the front pair of wings is copper;
however, this color may be lost as the moth loses its
scales. The inner half of the wing near the body is
light gray. The hind wings are gray and have no
distinctive markings.
Female moths deposit from 60 to 300 eggs, singly or
in groups on or within the upper surface of the
grain mass. The larvae move about in the upper
grain mass, feeding on fines and cracked kernels
and producing a silken webbing as they feed. Full
grown caterpillars may leave their food source and
climb up walls to pupate. The life cycle from egg to
adult takes about six to eight weeks during warm
weather. There are usually four to six generations
per year depending on food supply and
temperature.
The difficulty in controlling this pest is very dependent
upon how bad the problem is when you find it. If an
infestation is present but a mess of spoilage and silk is
not yet present, you may get control by applying a "cap-
out" treatment to the surface of the gain. A list of
insecticides labeled for use can be found in the annual
insect control recommendations for the appropriate crop.
If the mess is already present, then spoiled grain, and
insect mess must be removed before an application can be
made. Be sure to check for any crusting. As always
getting a handle on the situation before it gets bad is the
key to good economic control.
TOBACCO
 The Kentucky Blue
Mold Warning System's URL address is:
The Kentucky Blue
Mold Warning System's URL address is:
http://
www.uky.edu/Agriculture/kpn/kyblue/kyblue.htm
METHYL BROMIDE PHASEOUT CHANGES
(From NAPIAP Reregistration Notification Network, Oct.
28, 1998).
Due to recent legislative actions by the U.S.
Congress, the methyl bromide phase out in the U.S.
has been changed. Methyl bromide production and
importation will be reduced from 1991 levels as
follows:
- 25% reduction in 1999
- 50% reduction in 2001
- 70% reduction in 2003
- 100% reduction in 2005
Preshipment and quarantine uses exempt
Critical agricultural uses allocated after 2005.
Congress attached an amendment to the Fiscal Year
1999 (FY99) Appropriations bill that makes specific
changes to the Clean Air Act. The amendment will
require that the EPA make regulatory changes to the
U.S. phase out of methyl bromide. These changes
will essentially "harmonize" the U.S. phaseout of
methyl bromide with the Montreal Protocol
phaseout schedule for developed countries. This
schedule, agreed to by the Parties to the Montreal
Protocol in 1997, involves graduated reductions in
methyl bromide consumption (production plus
imports minus exports based on 1991 levels) and a
2005 phase out. In addition to these reductions in
methyl bromide consumption, the Parties to the
Montreal Protocol exempted from any control
measures quarantine and pre-shipment uses of
methyl bromide.
For more information see:
http://www.epa.gov/ozone/mbr/harmoniz.html
FUNGICIDES USED ON TOBACCO REQUIRE ADDITIONAL AND SPECIAL
TESTS BEFORE LABELING - RESPECT THIS!
By William Nesmith
There is concern about the amount of off-label
fungicides use by tobacco growers. One area of
special concern relates to the use of fungicides that
may be labeled for use on other crops (such as food
crops) but which are not labeled for use on tobacco.
Tobacco growers find this difficult to accept and
some resort to using a product on tobacco without a
label. They justify their actions with statements like
this: "If it is safe enough for tomatoes it must be safe
to use on tobacco".
Oh, how wrong is this position! For example, there
is one fungicide group being widely used legally in
tomatoes and many other food crops for disease
control that is NOT labeled for tobacco for very
good reasons. Why? When that fungicide is used in
tobacco for foliar disease control, the cured tobacco
during smoking releases toxic volatile compounds
that would cause immediate damage to the smoker.
There are other fungicides, also labeled on tomatoes
and other food crops, that present no known toxic
metabolites in the tobacco smoke, but their use in
tobacco production is unacceptable because they
result in off-favors (objectionable flavors) in the
smoke.
Therefore, before a fungicide is labeled for use in
tobacco production, special tests are required, over
and above what are needed for most other crop
uses. Some of those tests are: Smoke Inhalation and
Pyrolytic products Analysis, designed to evaluate
potential toxins produced in the smoke generated at
200, 400, and 800 C, temperatures normally
experienced during the burn associated with
cigarette smoking. Another important test is the
Smoke Panel Taste Evaluations of Cigarettes Made
from Experimental-Pesticide Treated Tobacco. This
smoke flavor test is usually the final test conducted
before labeling. It is designed to detect objectionable
or other off-flavors associated with the cigarettes
made from tobacco treated with the candidate
pesticide at rates being considered in the
proposed/expected label.
It is important for all in the tobacco industry to
understand that these special tests are usually not
conducted with the pesticide until after efficacy
testing (to determine pest control potential) has been
completed or at least is well underway. These
special tobacco tests are expensive and no pesticide
manufacture will conduct them, for economic
reasons, until they have abundant data to support
that the compound is efficacious and that a
favorable market window exists. Furthermore, all
must come to appreciate that a significant number
of pesticide compounds that are safe to use in other
crops FAIL to pass the critical additional tests
needed to protect the safe use of pesticides in
tobacco.
My advice to tobacco growers is to respect that they
are best served by using only fungicides specifically
labeled for their crop and site. Off-labeled use of
fungicides and other pesticides has the potential to
create serious problems for the tobacco industry.
FALL-FUMIGATION OF FLOAT-TRAYS
By William Nesmith
Several growers have tried fumigation of float trays
with methyl bromide as a means of tray sanitation.
Their results have been mixed, with some reporting
excellent success while others report failure.
Researchers from NC State University and the
University of Kentucky have demonstrated that
methyl bromide can be an effective means of
disinfecting float trays, especially for the control of
Rhizoctonia, but significant Pythium survives even
when fumigation efforts are ideal. So it is not the
perfect tray sanitation system that some want to
make it out to be.
In visiting with growers from both the success and
failure sides of this issue, the following points were
learned.
* Some have experienced personal injury, mainly to
feet, when removing the trays from their fumigation
chamber. Please urge all operators using this
product to read and follow the label. Fumigation of
contaminated soil and trays is implied under section
F of the methyl bromide label (Potting Mix
Fumigation Directions).
In many cases, these injuries occurred when using
fumigation chambers that were planned to be
reused, such as polytubes. Some occurred when the
chamber was located on unlevel sites. Because
methyl bromide is a heavier-than-air gas, it will
concentrate on the floor and move downhill. Use
level sites and be able to fully ventilate the entire
chamber before entry - including ALL of the floor.
* Low temperature during fumigation was a major
reason for failure. Most failures were associated
with fumigating during cold weather in February
and March.
The temperature in the fumigation chamber should
be 60F or above, at the coolest point.. It is difficult to
obtain such temperatures outside when fumigating
in February or March in Kentucky. Growers may
have more success in obtaining acceptable
fumigation temperatures if they make some
appropriate modifications. First, select an outdoor
site with southern exposure, away from the shade of
buildings, etc. Fumigate in the fall, when higher
temperatures are more likely. Clear plastic (at least
4 mils thick) should be used over the top to enclose
the trays and allow sunlight to penetrate and heat
the system. There may be an advantage to using
dark plastic on the bottom (floor) to absorb the heat.
Allow the system to reach 60F before releasing the
gas. Also, allow at least a 48-hour fumigation
period, before starting the aeration events.
* Growers substituting fumigation for all other
steps in tray sanitation usually reported failure,
while most cases of success involved washing the
trays followed by fumigation after the trays had
dried. A 10% bleach/fumigation event incorporated
before or after methyl bromide-fumigation has
proved beneficial in some studies.

VEGETABLES
SANITATION CONTROLS DISEASES IN THE HOME VEGETABLE GARDEN
By John Hartman
At the end of the growing season, often after the
first hard frost, it is time to clean up the garden.
Garden clean-up done well is an exercise in
sanitation, and is an excellent and effective plant
disease control practice. If not cleaned up, the
infected or contaminated remains of the previous
crop may provide an abundant source of disease-
causing microbes the next year.
Many disease-causing fungi and bacteria can live
over the winter on diseased roots, stems, leaves, or
fruits. These microbes survive in several different
ways:
Some fungi, such as those causing tomato early blight and powdery mildew survive until next
season on dead host plant tissues, even vegetation broken off and left behind as debris.
Others, such as downy mildew, an obligate parasite, may develop on live suckers growing
from stems and roots which, even with the tops cut off, can survive mild winters and allow the
pathogens to grow. In addition to obligate parasites, other pathogens could also survive or
even increase on such overwintering hosts. Even weeds such as winter annuals can harbor
diseases.
The gray mold fungus and the Rhizoctonia and Pythium root rot fungi can live as saprophytes
on garden plant debris.
Pathogens such as root knot nematode and the fungi causing Fusarium and Verticillium wilt
produce resting structures that can survive well in the soil even after the plant tissue has
completely decayed.
In autumn, remove all the plants (except cover crops
or winter vegetables) from the vegetable garden. Be
sure to carefully dig up the roots and take them
away as well. Roots left to decompose in the soil
can release microbes that will survive there. If
plants are not being removed, then till the garden in
the fall to break the dead plant material into smaller
pieces and to turn them under. Buried plant debris
decomposes faster than plant debris left on the
surface. This will reduce the population of disease-
causing microbes left in the garden to attack next
year's crop.
What is to be done with all this plant debris? If the
gardener has a good compost pile that heats up and
completely decomposes plant remains over a period
of a few years, most of the disease-causing
pathogens will also be destroyed. This, then,
completes the process of garden plant disease
control by sanitation. If heat development in the
composting process is not possible where there is a
concern about survival of root knot, or Fusarium
and Verticillium wilt pathogens, infected plant parts
should be removed from the garden and placed
where they would not be recycled back into the
garden.
HOUSEHOLD

THERE'S A HOLE IN MY SWEATER!
By Mike Potter
With the onset of cold weather, clients will be
calling about 'bugs' infesting their clothing and
other items unpacked from storage. These are
probably clothes moths or carpet beetles. Besides
damaging fabric, these insects will feed on any item
composed of animal fibers, e.g., wool, fur, silk,
feathers, felt or leather. Items commonly infested
include wool sweaters, coats, blankets, carpets,
down pillows and comforters, upholstered
furniture, toys and animal trophies. Synthetic fabrics
such as polyester and rayon are rarely attacked
unless blended with wool, or if they are heavily
soiled with food stains or body oils. Serious
infestations of clothes moths and carpet beetles can
develop undetected in a home, often causing
irreparable damage to clothing, bedding, rugs, and
other articles.
THE CULPRITS
Carpet beetles - Larvae are about 1/8 to 1/4-inch
long, tan to brownish in color, and densely covered
with hairs or bristles. This is the life stage likely to be
encountered now since only the larvae feed on
fabrics and cause damage. Oftentimes, only the shed
(molted) skins of the larvae are present on the
damaged item. Adult carpet beetles feed primarily
on flowers and are usually discovered indoors
during the spring. The adult beetles are small (1/16
to 1/8-inch) and oval-shaped, ranging in color from
black- to various patterns of white, brown, yellow
and orange. Large numbers may be spotted around
light fixtures and windows, indicating that an
infestation is present somewhere within the
structure.
Clothes moths- Clothes moths are small (1/2-inch),
buff-colored moths with narrow wings fringed with
hairs. Like carpet beetles, they damage fabric only in
the larval stage. Adult clothes moths are seldom
seen because they avoid light, preferring to hide in
dark places such as the backs of closets. Clients who
report seeing tiny moths in the kitchen and other
well-lighted areas are probably seeing grain moths
originating from stored foods, e.g., cereal, dried fruit,
nuts, or pet food. Clothes moth larvae spin silken
feeding tubes or patches of webbing as they move
about on the surface of fabrics. They also deposit
tiny fecal pellets similar in color to the fabric.
THE SOLUTION
Current infestations- Controlling an existing fabric pest
problem requires diligence and a thorough inspection to
locate all infested items and areas of infestation. The
source may be an old woolen scarf at the back of a
closet, a fur hat in a box, an unused remnant of wool
carpeting, or an abandoned bird or squirrel nest up
in the attic. Larvae prefer to feed in dark,
undisturbed areas where susceptible items are
stored for long periods. Larvae also may be found
living beneath the edges of carpeting (use needle-
nose pliers to lift the edge of the carpet from the tack
strip along baseboards), underneath and within
upholstered furniture, or inside heat ducts and floor
vents, feeding on accumulations of lint, pet hair and
other bits of debris. Occasionally, infestations may
originate from bird or animal nests or carcasses
present in an attic, chimney or wall void. Carpet
beetles, in particular, will also feed on pet food, bird
seed, and cereal products associated with the
kitchen, basement or garage.
Infested items should be laundered, drycleaned or
thrown out. Laundering (hot cycle) or drycleaning
kills any eggs or larvae that may be present.
Vacuuming floors, carpets, and heating vents
effectively removes larvae as well as hair and lint
which could support future infestations. Be sure to
vacuum the edges of carpets, along baseboards,
underneath furniture and stored items, and inside
closets and 'quiet' areas where carpet beetles and
clothes moths prefer to feed.
Insecticides applied to infested areas may be helpful
as a supplement to good housekeeping. Products
containing active ingredients labeled for flea control
(e.g., permethrin) are effective. Sprays may be
applied to carpets, especially along and beneath
edges adjacent to baseboards, underneath furniture,
and other likely areas of infestation where
prolonged contact with humans is unlikely.
Clothing and bedding should not be sprayed with
household insecticides and should be removed
before treatment.
Avoiding future problems- The best way to avoid
future problems with fabric pests is through pre-
vention. Woolens and other susceptible fabrics
should be drycleaned or laundered before being
stored for long periods. Cleaning kills any eggs or
larvae that may be present and also removes
perspiration odors that are attractive to the pests.
Articles to be stored should then be packed in
tight-fitting containers with moth balls or flakes
containing paradichlorobenzene (PDB) or
naphthalene. The vapors from these materials are
only effective if maintained at sufficient
concentrations. Effective concentrations can best be
achieved by sealing susceptible items (with the
manufacturers' recommended dosage of moth
crystals) in large plastic bags, and then storing the
bagged articles in tight-fitting trunks, boxes or
chests. Contrary to popular belief, cedar closets or
chests are seldom effective by themselves because
the seal is insufficient to maintain lethal or repellent
concentrations of the volatile oil of cedar.
Conventional household insecticides should not be
used to treat clothing; however, mothproofing
solutions may be applied to susceptible clothing by
professional dry cleaners. Valuable garments such
as furs can be protected from these pests by storing
them in cold vaults a service offered by some
furriers and department stores.
Additional tips on fabric pest prevention, control,
and repair of damage can be found in the
publication IP-50, Fabric Insect Pests. Elimination of
widespread, persistent infestations of carpet beetles
and clothes moths in a home or commercial
establishment may require the services of a profes-
sional pest control operator.
DIAGNOSTIC LAB HIGHLIGHTS
By Julie Beale and Paul Bachi
Most of the recent laboratory samples have been
from the landscape. We are seeing powdery
mildew on euonymus, oak, lilac, etc., plus stress
symptoms on many landscape trees,
particularly conifers. One symptom, often confused
with stress, is the normal yellowing and loss of last
year's needles on white pine. We have also seen
Volutella blight on pachysandra and Phoma stem
blight on vinca.
Disease problems of greenhouse ornamentals have
included Colletotrichum leaf spot on pansy and
Pythium root rot on pansy and poinsettia. Leaf
mold on greenhouse tomatoes was also observed
last week.
PESTICIDE NEWS AND VIEWS
By Lee Townsend
From the American Crop Protection Association.
FQPA SPOTLIGHT/OCTOBER 30, 1998/VOLUME 2, ISSUE 21
EWG PULLS OUT OF TRAC AND PPDC
In an October 26 letter to Vice President Gore, the
Environmental Working Group's Ken Cook announced
the resignation of EWG from both the Tolerance
Reassessment Advisory and Pesticide Program Dialogue
Committees. EWG earlier had threatened such action
when TRAC Co-Chairs Rominger (USDA) and Hansen
(EPA) extended the life of TRAC for two more meetings
to be held in early '99. In his letter, Cook complained
that, although "EWG staff members...have devoted
hundreds of hours to prepare for or attend TRAC and
PPDC sessions...we cannot point to any tangible
[administration] action to actually protect children from
pesticides." He also deplored the administration's
acceptance of recent legislation to delay the phase-out of
methyl bromide, and its unwillingness to "act to reduce
[pesticide risks] in deference to economic concerns of
agribusiness groups, pesticide companies and food
processors."
ACPA, in comments to members and allies, pledged to
continue to work with the TRAC process and echoed the
feelings of most TRAC members that past meetings have
been worthwhile with all stakeholders given ample time
to express views in open, transparent sessions. Most
notably, the TRAC process has resulted in the public
comment opportunity on soon-to-be-released policies (see
next item) that are key to full and fair FQPA
implementation. Such policies, if based on sound
science, will lead to increased public confidence and, in
some cases, further improvements in food safety for all,
including children. In the meantime, ACPA noted that
the current pesticide regulatory system is protective of
public health and specifically evaluates potential risks to
children.
NOTICE ISSUED ON SCHEDULE AND FRAMEWORK FOR FQPA POLICIES
Published in the October 29 Federal Register was a
schedule and framework for issuance of a series of nine
science policies to implement FQPA provisions. (The
policy issues are outlined in TRAC staff paper #26.)
EPA noted that the action was a direct result of TRAC
discussions and that comments on each interim science
policy document will be invited through separate notices
in the Federal Register, as outlined in the framework.
The document, "Framework for Addressing Key Science
Issues Presented by the Food Quality Protection Act as
Developed Through the Tolerance Reassessment
Committee," is available on ACPA's fax on demand
(888/587-0438) as document #254. EPA /OPP contact:
Jeff Kempter, (703) 305-5448; kempter.carlton@epa.gov.
WESTERN STATES ZERO IN ON NONFOOD USES
An effort to remove a number of non-crop pesticide uses
from consideration under FQPA has been launched by the
Risk Assessment Subcommittee of the Western States
FQPA Coalition. The subcommittee is preparing an issue
paper that will ask EPA to remove nonfood and nonfood
type uses from risk cup calculations. Nonfood uses
include crops grown for seed, ornamental nursery stock,
sod production, and seed treatments, among others. Key
to the position is that nonfood uses do not pose a dietary
risk and their elimination would allow for more efficient
implementation of FQPA.
ENVIRO STUDIES GAIN LITTLE NEWS MEDIA COVERAGE
Two recent reports, one by Environmental Working
Group on chemically tainted Ohio drinking water, and
one by Natural Resources Defense Council on pesticide
effects on children on or near farms, appear to have
gained little news media coverage. One Ohio account
noted some skepticism related to EWG's water study. A
telling comment by EWG head, Ken Cook, may explain
why. He admitted to the study having "very weak data as
to what's in the water." And, he noted that, because of the
high $1,500-per-sample cost, EWG could afford only one
sample for each of the dozen Ohio communities covered.
As for NRDC's report, many farm families may feel the
same as Sheila Massey of Women Involved in Farm
Economics (WIFE). As she told the TRAC group
recently, "We have raised three extremely healthy
children [who] have lived around [and helped with]
pesticide application their entire lives, and none has had a
health related problem due to pesticide exposure." Her
comment points up the fact that pesticide use does not
mean pesticide exposure.
FQPA Spotlight is published biweekly by the American
Crop Protection Association, 1156 15th St., NW,
Washington, DC. Don Collins, Margaret
Speich, editors; (202) 872-3863; don@acpa.org;
margaret@acpa.org.
MISCELLANEOUS
PESTICIDE TRAINING MEETINGS
By Lee Townsend
Training and Testing Schedule
Lee Townsend
Extension Entomologist
BACK
TO KY PEST NEWS HOME
 Often,
the most common storage problem is sprouted
grain, especially when rainfall follows a long dry
spell. Detecting both of these problems can be
relatively simple. Open the top bin hatch and look
and SMELL inside. Be sure to do this before even
considering entering the bins. (Which by the way
can be very dangerous. Make sure you know what
you are doing. Cut the power to the bins and have a
partner BEFORE entering a bin). A foul or musty
odor is a very good tip that something is wrong.
Often,
the most common storage problem is sprouted
grain, especially when rainfall follows a long dry
spell. Detecting both of these problems can be
relatively simple. Open the top bin hatch and look
and SMELL inside. Be sure to do this before even
considering entering the bins. (Which by the way
can be very dangerous. Make sure you know what
you are doing. Cut the power to the bins and have a
partner BEFORE entering a bin). A foul or musty
odor is a very good tip that something is wrong.

