In last week's Kentucky Pest News, I discussed the concern over potential turf injury when DMI fungicides are used in conjunction with a turf growth regulator with a similar biochemical mode of action, namely Scott's TGR (paclobutrazol) or Cutless. In the article, I inadvertently identified triexapac-ethyl as the active ingredient in Cutless. The active ingredient in Cutless is flurprimidol. Triexapac-ethyl is the active ingredient in the turf growth regulator, Primo. Primo is not reported to interact with DMI fungicides to enhance injury potential to the turf. Please accept my apology for any confusion this may have caused.
July 25, 1999, KY Dam Village Convention Center, Gilbertsville, KY
See the details on the Pesticide Applicator Training Web Site http://www.uky.edu/Agriculture/PAT/welcome.htm

Weather conditions much of the coming week are expected to provide near ideal conditions for blue mold to develop where viable spores land on susceptible tissues. Susceptibility of the plant will vary greatly, however, from field to field and even from leaf to leaf on the plant. Where abundant water is now available and succulent growth is occurring, the new growth will be highly susceptible, especially with the cloudy weather being experienced. The volume of blue mold spores available should be low in most areas of Kentucky, so slow increase in blue mold activity is expected. The sudden emergence of damaging levels of disease over a large area is unlikely at this time, but the longer the wet, cloudy weather persists the sooner we could experience such high risk potential. But, all sites are not at equal risk. In some fields and communities (irrigated crops or in regions and communities that have received significant or timely rains) humid nights and crops of very rapidly growing tobacco on wet sites could sustain damaging level of blue mold where local sources of viable spores are nearby. However, in most communities, the numbers of spores available should be very low resulting in only scattered lesions developing even in highly susceptible crops. The greatest current potential for high levels of viable spores would be from float greenhouses with active disease and from fields of tobacco that had active disease present last week.
Blue mold has been confirmed this season in the counties of Clay, Fayette, Hardin, Marion, Nicholas, and Woodford. Active blue mold has also been confirmed nearby in Montgomery and Robertson counties in north west Tennessee (near the Todd County, Kentucky line). WARNINGS exist within five miles of the communities with blue mold. Local County Extension Offices are the best sources for information of the specific communities with blue mold. WATCHES exist for all of these counties plus the adjacent counties.
In southwestern Kentucky, the WATCH is expanded to include the counties of Logan, Todd, and Christian, because significant blue mold was discovered nearby in Montgomery and Robertson counties of Tennessee. Dr. Steve Bost, University of Tennessee in Nashville reported on June 28 that significant spread probably has occurred late last week and during the weekend from two moderate outbreaks in that region. Development of blue mold in this border region could be much more rapid than the rest of Kentucky, because of rapid plant growth, adequate moisture, and significant spore load. This is a particularly critical area, relative to Kentucky, because activity in this region is strategically located where prevailing winds can send spores rapidly to most other major production areas. Therefore, pay attention to developments in this region.
Active blue mold has also been confirmed in the burley production of western North Carolina (Buncombe, Madison, and Yancey counties). Remain alert to the blue mold development in this mountain region, even though it is far away, because this area is more likely to experience favorable weather for blue mold than most other areas during mid summer and can provide a large mass of spores to Kentucky.
Tobacco growers should keep spray equipment properly maintained and remain alert to the current status of blue mold in the state and community, then adjust rapidly should the needs change. Foliar fungicide sprays are recommended for communities under a watch or warning and all irrigated crops. A major effort should be made to destroy ALL transplants not being specifically maintained (with fungicides) for late plantings, lest they become sites to harbor buildup of blue mold.
Since we are experiencing a season with much lower threats from blue mold than in recent years, several have asked for more guidelines about timing of fungicide applications. When sprays are needed, it is important that they be applied in a manner to provide good coverage and often enough to keep up with new growth and wash-off. Poor coverage or poor timing of coverage, means poor control. Be sure to follow labels carefully and adjust fungicide level and water/spray volume to the stage of crop growth and developments, and use appropriate nozzles, and adequate pressure. In timing fungicides, consider the following four options:
1) The least risky is to begin sprays after transplanting and repeat sprays weekly, regardless of whether the conditions are favorable for blue mold development. This approach provides the highest potential for controlling blue mold, but it has the disadvantage of resulting in excessive and unwarranted sprays. Furthermore, this approach is not supported by either of the labeled products, Acrobat MZ or Dithane DF, in Kentucky. The product labels clearly state to begin applications when conditions favor blue mold development and to discontinue sprays when the threat of blue mold no longer exists.
2) A second option is to wait and begin sprays when a blue mold watch or specific advisory is issued for the community. A watch is issued when conditions are believed to be favorable for blue mold development in the area and a source of viable inoculum (spores) is believe to be impacting the area. This is a very sound approach and it is what is recommended by the University of Kentucky, but it requires an alert grower that is receiving the latest information from the Kentucky Blue Mold Warning System. Delaying sprays until a watch has been issued worked very well in University tests and for many growers, but it has often failed at the farm- level, because growers react too slowly or without adequate spray equipment. When major epidemics of blue mold are occurring in the region so that a large mass of spores is arriving, growers must react quickly and well to the advisory. A classic example of this was in mid July of 1996 when a major epidemic of blue mold was occurring in the Bluegrass Region of central Kentucky and a watch was posted for southern and south central Kentucky, which clearly stated that a massive spore- load was expected to move into the region out of the Bluegrass. Few growers sprayed in south central Kentucky at the time of the watch and the first blue mold they observed was 50% of the leaf surface spotted.
3) The third option, is to wait to begin sprays when a blue mold warning is present in your community. Warnings are issued when blue mold is confirmed in an area and conditions are expected to remain favorable for continued development. This option also has all the risks associated with option #2, plus it is possible that the disease will not be detected until after several cycles and the disease has become widespread.
4) The fourth option is to wait to begin sprays after the disease is in the field. This is a very risky approach and it is not recommended, because it can result in economic losses and failure to provide effective control, unless one is scouting the field carefully and often, and starts the sprays when only a few lesions are in the field.
![]() The Kentucky Blue Mold Warning System's web address is:
The Kentucky Blue Mold Warning System's web address is:
http://
www.uky.edu/Agriculture/kpn/kyblue/kyblue.htm
 Tomato Spotted Wilt Virus (TSWV), which is
vectored by thrips, is becoming more prevalent,
both in range of distribution and incidence of the
disease. However, overall incidence and losses are
still very low in Kentucky. Major epidemics have
occurred in some flue-cured tobacco regions of the
southeastern USA. A related Tospovirus, Impatiens
Necrotic Spot Virus, (INSV) may also be involved,
but it has been detected in only a few cases on
tobacco in Kentucky. INSV usually causes only spot
in tobacco, but some strains can cause serious
damage in the field in late summer. The Tospovirus
disease complex is rapidly becoming one of the
most important disease in the US on many crops,
possibly related to range expansion of certain thrips,
which vector the virus much more efficiently than
native thrips and the increased use of greenhouse
transplants.
Tomato Spotted Wilt Virus (TSWV), which is
vectored by thrips, is becoming more prevalent,
both in range of distribution and incidence of the
disease. However, overall incidence and losses are
still very low in Kentucky. Major epidemics have
occurred in some flue-cured tobacco regions of the
southeastern USA. A related Tospovirus, Impatiens
Necrotic Spot Virus, (INSV) may also be involved,
but it has been detected in only a few cases on
tobacco in Kentucky. INSV usually causes only spot
in tobacco, but some strains can cause serious
damage in the field in late summer. The Tospovirus
disease complex is rapidly becoming one of the
most important disease in the US on many crops,
possibly related to range expansion of certain thrips,
which vector the virus much more efficiently than
native thrips and the increased use of greenhouse
transplants.
This disease was first reported from Kentucky in 1986, when low incidence of infections were scattered about 30,000 acres of tobacco and tomatoes in south central Kentucky. Since then, it has occurred every year and has been diagnosed from nearly every Kentucky county, but mostly confined to a few plants in the field. The level of TSWV in Kentucky's tobacco is still low, overall, and usually cannot be detected but there are a few cases in tobacco where significant crop damage is beginning to occur, mainly in southern Kentucky. In most fields with TSWV the incidence is confined to a few scattered plants, well below 1% of the plants. However, in some areas of southern Kentucky, reservoirs of TSWV and its thrips vectors appears to have become established in and around certain field borders, probably on broadleaf weeds. In such fields the incidence of disease is at the point that economically damaging levels are present, with worse-case incidence ranging from 15-30% of the plants infected and destroyed. On most farms with serious problems, the incidence has increased gradually over about a 10-year period.
Infection of TSWV may occur at any stage of plant growth, with symptom timing of development and severity following inoculation being highly variable. Several strains of the virus may be involved and because plant age, plant development, and environment affect symptom development. Some strains only develop symptoms under high temperatures, others following rapid growth, while others following stress or even with the onset of flowering. I have observed symptoms in the greenhouse within 4-7 days of inoculation, but when newly inoculated transplants have been set to the field prior to showing symptoms, symptom development in the field has ranged from a week following transplanting to flowering time. Initial infection may occur in transplants or the field settings, but more often in Kentucky it has been connected with introduction of infected transplants. On problem farms in southern Kentucky, early planted crops have the highest incidence, in general. This virus complex is very damaging to the plants that become infected, usually leaving the infected plant useless or dead, but there is much variation.
A wide range of symptoms are present on tobacco, which overlap with those caused by Tobacco Ringspot Virus, Tobacco Streak Virus, Fusarium Wilt, Soreshin and Systemic Blue Mold, so diagnosis requires very careful observations and special tests in the lab. Initial symptoms of necrotic spots along the veins and concentric or zonate spots on the leaf, followed by obvious yellowing, stunting and wilt, are the most prevalent symptoms noticed in Kentucky. In time, necrotic lesions become very obvious on the stem and sometimes within the pith. The vascular system is often stained brown, but not as extensive as with Fusarium wilt. The affected leaves often become distorted and may pucker due to unequal growth on one side. The apical bud droops or bends to one side and is followed by wilting of most of the foliage on that side of the plant. Many plants will not die; instead, they sucker and produce a very stunted and bushy growth with many spots on small unmarketable leaves. If soreshin or black shank are active at the site, TSWV- infected plants often are killed by these other diseases.
Spread of the virus is through thrips, and several species of thrips are known to vector the virus. However, preliminary findings suggest that the western flower thrips, a relatively new arrival to the state, is a much more efficient vector than the more common thrips in the state. The virus is spread by the thrips in a persistent fashion, which means that once the vector has acquired the virus, it can transmit for a long time. The juvenile thrips (nymphs) acquire the virus while feeding on infected plants, but these juveniles cannot spread it to healthy plants. Instead, only the adult stage can transmit the virus, which it can do for the remainder of its life. This causes a delay of 3-18 days between the acquisition of the virus by the nymphs and the transmission of the virus by the adults. Adult thrips do not acquire the virus, however. Because the thrips move about the field in short flights, the distribution of infected plants from inoculations by the same insect usually leaves a clustered-pattern, where disease plants fit into small groups of 3-10 nearby plants, involving multiple rows of tobacco. Such a clustered pattern in the field would suggest infection occurred after the plants were set, while a totally random pattern or clusters within a row (plants set from the same tray of plants) usually would suggest the transplants were infected prior to transplanting. With containerized transplants, we can see a strong tray-effect to the distribution, several plants clustered in the same row while adjacent rows are free of infection or with a very different rate of infection. Highest incidence of infected transplants have been associated with transplants produced in greenhouses that also contain ornamentals, especially hanging baskets of flowers located above the transplants.
Control of TSWV has been difficult in areas of the world where it is prevalent. The variables involved in Kentucky outbreaks of this disease, as well as many of those elsewhere in the U.S., are still unknown. Several laboratories are working with this disease in tobacco. Evidence obtained from field observations over the past several years in Kentucky suggests that most infections appear to result from either transplanting infected transplants and/or heavy influx of virus-carrying thrips from outside the field. The earliest set crops seem to have the greatest level of TSWV. We see very little evidence to support secondary spread (from tobacco to tobacco) within the planting, although the possibility certainly exists if the thrips are able to complete their life cycle in an infected field.
Adult thrips are less than 1/16 inch long. Color varies with the species, ranging from blue to light yellow. Thrips lay their eggs inside the plant tissue where the eggs are protected until they hatch and emerge 3 to 4 days later. Early stages of the immatures usually are clear to light yellow and hide in the bud and flower parts of a plant. There is a resting stage that occurs in the soil or leaf litter prior to metamorphosis to an adult. Adults emerge from the soil 2 to 5 days later and may be yellow or dark brown. They hold their four hair-fringed wings flat over their backs. Adults can live 30 to 45 days.
At this point, it is unclear as to whether attempts to control thrips within the field planting will significantly reduce disease activity in Kentucky field plantings. However, we suggest normal insect control programs be maintained, especially in transplant production. Rogueing out infected plants should be considered where the plants were introduced from off-the-farm or other states to minimize the risk of the virus becoming established locally. If the disease is found in transplant operations, destroy the infected plants as soon as possible. Do not grow tobacco and ornamental in the same operation.

As the crop progresses this season, the types of pests
attacking corn changes.
 Corn rootworm beetles have
begun to emerge from corn fields and need to be
monitored over the next few weeks. Monitoring of
rootworm adults serves two purposes, first corn
rootworm adults can interfere with pollination if silk
feeding is severe prior to and during pollen shed. In
addition, beetle counts this year are used to determine
the need for a soil insecticide next year if corn is to be
replanted in the same field.
Corn rootworm beetles have
begun to emerge from corn fields and need to be
monitored over the next few weeks. Monitoring of
rootworm adults serves two purposes, first corn
rootworm adults can interfere with pollination if silk
feeding is severe prior to and during pollen shed. In
addition, beetle counts this year are used to determine
the need for a soil insecticide next year if corn is to be
replanted in the same field.
Japanese beetles
 and corn rootworm beetles feed on
corn silks and can, potentially, interfere with
pollination. Generally, silk feeding by these beetles
does not reduce pollination because they often cut the
corn silks after pollination has already taken place. If
corn silks are cut prior to pollination, they will
continue to grow. However, if two or more Japanese
beetles or five or more corn rootworm beetles are
present per ear and silks are clipped to less than 1/2
inch prior to pollen shed, then treatment may be
required.
and corn rootworm beetles feed on
corn silks and can, potentially, interfere with
pollination. Generally, silk feeding by these beetles
does not reduce pollination because they often cut the
corn silks after pollination has already taken place. If
corn silks are cut prior to pollination, they will
continue to grow. However, if two or more Japanese
beetles or five or more corn rootworm beetles are
present per ear and silks are clipped to less than 1/2
inch prior to pollen shed, then treatment may be
required.
Corn rootworm beetle counts are used to determine the need for soil insecticides in continuous corn next year. The numbers of beetles are recorded from a group of twenty consecutive plants in each of a minimum of five locations per field. If an average of 1 or more rootworms beetles per plant is observed at anytime during the next 4 to 5 weeks, then a soil insecticide next spring is necessary if corn is to be grown in the same field next year.
 Fall armyworm is active in some late-planted fields
but I have not had reports of any treatable
infestations. Corn producers using Bt-corn hybrids
need to realize that, at best, Bt corn has very limited
control of fall armyworm. These producers should
pay particular attention to late planted Bt-corn fields.
Last year, a UK test plot in Lexington showed that the
YieldGard and NatureGard Bt corn suppressed fall
armyworm activity, but additional treatments may be
needed with heavy infestations.
Fall armyworm is active in some late-planted fields
but I have not had reports of any treatable
infestations. Corn producers using Bt-corn hybrids
need to realize that, at best, Bt corn has very limited
control of fall armyworm. These producers should
pay particular attention to late planted Bt-corn fields.
Last year, a UK test plot in Lexington showed that the
YieldGard and NatureGard Bt corn suppressed fall
armyworm activity, but additional treatments may be
needed with heavy infestations.
 Southwestern corn borer has been active in the
western portion of the state, particularly in some parts
of Daviess, McLean, Henderson and Union counties.
Some of the larvae have begun to bore into stalks, but
all ages are present in some fields. Late planted non-Bt
fields are the most vulnerable. Bt corn should resist
attack by the first generation, but only the YieldGard
and StarLink will reliably control the next generation.
Corn planted after May 1 is at greater risk to this pest.
Southwestern corn borer has been active in the
western portion of the state, particularly in some parts
of Daviess, McLean, Henderson and Union counties.
Some of the larvae have begun to bore into stalks, but
all ages are present in some fields. Late planted non-Bt
fields are the most vulnerable. Bt corn should resist
attack by the first generation, but only the YieldGard
and StarLink will reliably control the next generation.
Corn planted after May 1 is at greater risk to this pest.
![]()
Japanese beetles (JB) are a familiar soybean feeder in central Kentucky from late June through early August. However, they have moved west to the Mississippi River. Although not a common problem in western KY, you should be on the look out for them. Japanese beetles can be found in very large numbers on soybeans. Infestations often begin as the beetles feed first on smartweed and then move over to beans. The importance of this pest should also be viewed with corn in mind, as they are also silk feeders. Moderate populations in soybeans at the time of corn 'silking' may be more important to corn than the numbers in the beans would suggest.
 Japanese beetles are metallic green and bronze
beetles about 3/8" long. There is a row of white tufts
on the side of the body below the bronze wing
covers. The 'goldsmith' beetle is often mistaken for
the JB. However, the goldsmith beetle does not
have the row of white tufts on the sides.
Additionally, while the JB has very distinct areas of
green and copper colored areas on the body the
goldsmith beetle is generally bluish green all over
the body.
Japanese beetles are metallic green and bronze
beetles about 3/8" long. There is a row of white tufts
on the side of the body below the bronze wing
covers. The 'goldsmith' beetle is often mistaken for
the JB. However, the goldsmith beetle does not
have the row of white tufts on the sides.
Additionally, while the JB has very distinct areas of
green and copper colored areas on the body the
goldsmith beetle is generally bluish green all over
the body.
Generally when JB are causing an economially
important problem there will be so many of them
around that you need not count them. Treatment
decisions are based on defoliation of the soybean
plant. Controls should be considered if 30%
defoliation occurs during the vegetative stage, 20%
defoliation during reproductive stage.
A more
precise defoliation table which considers plant
stage, defoliation levels and crop value can be found
in IPM-3, KY-ICM manual for Soybean and ENT-
13, Insecticide Recommendations for Soybean.
Parades of insects in scarlet and black uniforms recall the days when "redcoats" marched through the Colonies. In this case, the culprits are the 1/8 to 1/4 inch long red and black burrowing bug nymphs. A true bug with sucking mouthparts and incomplete metamorphosis this insect has been seen in and around no-till soybean fields, as well as in corn fields. This species uses its sucking mouthparts to feed on sap from the roots of a wide variety of plants. They can be seen crawling over crop residue or accumulating in cracks in the soil surface.
There is no indication that burrowing bugs cause any injury to crops but densities of several dozen of these bright insects per square foot have raised the concern of farmers, dealers, and commercial applicators. In some cases, migrating burrowing bugs have covered the sides of buildings.
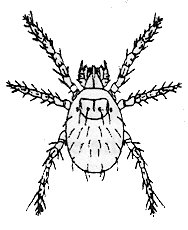 Chiggers are the larvae of a family of mites that are
sometimes called redbugs. Adults are large red
mites that can be seen running over pavements and
lawns. Chiggers are extremely small (0.5 mm). They
occur outdoors in low, overgrown damp places.
These mites feed on a variety of wild and domestic
animals, as well as humans. Wild blackberry
patches and grassy woods margins also are mite
territory because of they harbor many small rodents
that are good hosts.
Chiggers are the larvae of a family of mites that are
sometimes called redbugs. Adults are large red
mites that can be seen running over pavements and
lawns. Chiggers are extremely small (0.5 mm). They
occur outdoors in low, overgrown damp places.
These mites feed on a variety of wild and domestic
animals, as well as humans. Wild blackberry
patches and grassy woods margins also are mite
territory because of they harbor many small rodents
that are good hosts.
Chiggers overwinter as adults in the soil, becoming active in the spring. Eggs are laid on the soil surface. Newly hatched larvae crawl about until they locate and attach to a suitable host. Larvae feed for about 4 days and then drop off and molt to nonparasitic nymphal stage and ultimately the adult. The life cycle (from egg to egg) is completed in about 50 days.
Chigger bites commonly occur around the ankles, waistline, armpits, or other areas where skin is tender or where clothing fits tightly. These mites do not burrow into the skin but feed at pores or hair follicles. A salivary fluid is injected which dissolves cell tissue and produces a hardened, raised area around the site. Lymph, dissolved body fluids, and stray blood cells are withdrawn from the host through a feeding tube.
Most people react to chigger bites by developing an itch within 4 to 6 hours and dome-shaped reddish welts within 24 hours. Intense itching accompanies the welts, which may persist for 7 to 10 days. Besides causing intense itching, chigger bites that are scratched may become infected. Chiggers in North America are not known to transmit disease.
People who suspect they may have been attacked by chiggers should take a soapy bath immediately and apply antiseptic to any welts. A local anesthetic will provide temporary relief from itching.
Persons walking in chigger-infested areas can be protected by treating clothing (cuffs, socks, waistline, sleeves) or exposed skin with tick repellents. Some repellents should only be used on clothing; and it is important to follow label directions.
Regular mowing and removal of weeds and brush make areas less suitable for chiggers and their wild hosts. Mowing also enhances penetration and performance of miticides, should they be required. Chigger populations can be further reduced by treating infested areas with residual miticides. Applications should be thorough but restricted to areas frequented and suspected of being infested.
For more information, see Entfact-630 "Chiggers"
As was observed last year, maples in many Kentucky landscapes are not faring well. We are receiving laboratory specimens and getting numerous reports of maple trees or parts of trees dying statewide. The problem most often involves well-established Norway, sugar, and red maples. Homeowners usually report the sudden wilting and death of their trees; in some cases this is true, but in others, twig growth and tree ring analysis suggest that many of the dying maple trees have not been growing well for some years.
Although some of this information appeared in KPN last year, our purpose now is to review some of the causes of maple problems so County Extension Agents and landscape maintenance persons can better serve their clientele. In addition, Agents need to be aware of our diagnostic limits - one cannot diagnose girdling roots, mechanical injury, root rot, and usually Verticillium wilt with a sample consisting of some twigs, leaves, and small branches. However, site visits and perceptive questions of the growers are most useful, and for cases of mechanical injury, girdling roots, and inadequate planting site, would be more informative than a laboratory specimen.
There does not seem to be a single cause for the decline and death of landscape maples this year. We have observed a number of factors that have caused death or triggered decline and death of maples, including:
Girdling roots are probably the leading cause of decline, especially among Norway maples. Offending roots may not be visible above ground, but if the tree trunk does not have the normal buttress root flare at the base, and instead, goes straight into the ground like a telephone pole, self-girdling roots can be suspected. Trees with girdling roots may decline over a period of years, but then may collapse suddenly.
Verticillium wilt may infect all types of maples, and can also cause disease in tuliptrees, catalpas, golden-rain trees, and redbuds. Often developing on one side of the tree first, branches progressively wilt and die throughout the tree during the growing season. Where infections occurred late in the previous season, trees may not have even leafed out this year, or if they did, they immediately died. Mild winter temperatures may have allowed this soil-borne fungus to be more active than usual.
Canker and collar rot. We have diagnosed some cases of Phytophthora bleeding canker and collar rot on maples in past years. Trunks of affected trees have water-soaked bark spots. Collar rot, causing bark decay and wood staining, if well developed, can cause death of the top of the tree. Usually, collar rots and bleeding cankers lead to gradual decline of infected trees. The fungus Phytophthora is favored by high soil moisture levels, especially temporary flooding.
Restricted rooting space. Sugar maples planted as street trees sometimes lack space for their roots to exploit. Such trees with inadequate root systems would be especially vulnerable to drought and temporary flooding stresses.
Soil compaction from foot traffic, construction, or other activities crushes small roots and makes soils impervious to invasion by new roots. Affected maples may decline.
De-icing salts should not be a factor this year due to the mild winter.
Mechanical injuries. Construction such as laying utilities severs roots and triggers decline. Wounds to the trunk or large branches can also have negative effects on maple tree health.
Opportunistic fungi. Root, butt, and trunk rotters such as Ganoderma lucidum are found on some declining trees. In addition, canker and canker-rot fungi such as Botryosphaeria obtusa, Nectria cinnabarina, Cerrena unicolor, and Stegonosporium pyriforme are capable of invading weakened trees and causing branch dieback.
Although some infectious diseases are involved in the current wave of maple declines and death, much of the problem lies with urban stresses. In addition, recent hot weather, dry spring conditions, mild winter temperatures, drought late last summer, and any number of weather extremes from several years ago could be involved. In almost all cases, there is no reversing the decline. For those with still-healthy maples, continue to provide good growing conditions and be observant for the first indications of maple distress such as leaf scorch, premature fall color, and branch tip dieback.
 The fall webworm feeds on almost all shade, fruit
and ornamental trees except for evergreens. In
Kentucky some of the preferred trees include
mulberry, American elm, maples, hickory, and
sweetgum. Their tents are spun on the end of
branches and there is usually more than one
generation each year.
The fall webworm feeds on almost all shade, fruit
and ornamental trees except for evergreens. In
Kentucky some of the preferred trees include
mulberry, American elm, maples, hickory, and
sweetgum. Their tents are spun on the end of
branches and there is usually more than one
generation each year.
The fall webworm caterpillar is about one inch long, very hairy and is pale green or yellow. They may have either a red or black head. The blackheaded larvae have black spots along its back while the redheaded have orange to reddish spots. The blackheaded larvae will create a flimsy web while the redheaded makes a larger, more dense web.
Destroying the tents is an effective way of getting rid of the caterpillars on small trees. It is best to do this around dusk or early morning when the larvae are in the tent. If an insecticide is used, treat when the larvae are small and easiest to control, not when they are full grown and have already done their damage. Insecticides containing the active ingredient derived from Bacillus thuringiensis are the best choices for safety and selectivity. This is a stomach poison which must be ingested by the caterpillars. Treat foliage around the nest area. Applications to the tent or insects will not be effective. Do not burn the tents because the fire and intense heat may damage the tree or start a larger fire. Generally, there is no reason to control this insect on large, established trees.
 Many products are registered to control flies (horse
flies, stable flies, faces flies) on horses. Some are
sold as general livestock animals sprays that include
beef and dairy cattle, swine, and horses. Others are
the specialty products sold primarily to horse
owners. Usually both sets of insecticides are based
on the same active ingredients. While the product
list for horses is very long, the major ingredients are
pyrethrins, often with the synergist piperonyl
butoxide, synthetic pyrethroids (permethrin,
fenvalerate, cyfluthrin) or organophosphates
(coumaphos). They may be available in sprays,
wipe-ons, halter strips, or dusts. The synthetic
pyrethroid products tend to have the longest
residual activity of the three classes.
Many products are registered to control flies (horse
flies, stable flies, faces flies) on horses. Some are
sold as general livestock animals sprays that include
beef and dairy cattle, swine, and horses. Others are
the specialty products sold primarily to horse
owners. Usually both sets of insecticides are based
on the same active ingredients. While the product
list for horses is very long, the major ingredients are
pyrethrins, often with the synergist piperonyl
butoxide, synthetic pyrethroids (permethrin,
fenvalerate, cyfluthrin) or organophosphates
(coumaphos). They may be available in sprays,
wipe-ons, halter strips, or dusts. The synthetic
pyrethroid products tend to have the longest
residual activity of the three classes.
Often, the frustration from lack of fly control is due to the behavior and large numbers of flies rather than a lack of efficacy. The most troublesome species, such as face flies and horse flies, move over large distances and spend only short periods on the animals. There is a continual turnover of replacement flies so that it seems that nothing works very well. The products that are applied either kill the insect, or in the case of the synthetic pyrethroid insecticides, are very irritating to the flies, and they leave shortly after landing on the animal. Treated animals still draw flies. The protectant effect only comes into play once the insect lands. Consequently, treated animals will still be annoyed by flies to some extent, even when the "best" materials are used.
Last week in the diagnostic labs we saw Fusarium root rot, bacterial top rot, corn rootworm damage and zinc deficiency on corn and Rhizoctonia stem rot and brown spot (Cercospora) on soybean. On tobacco we diagnosed black root rot, black shank, soreshin, Fusarium basal stem rot, Fusarium wilt, three new cases of blue mold, tobacco streak virus, tobacco ringspot virus, tomato spotted wilt virus, lightning injury, stinkbug injury, manganese toxicity, phosphorus and potassium deficiency, frogeye leaf spot and frenching.
On fruits we have seen black rot of grape; cedar-apple rust and Phytophthora collar rot on apple; and Leucostoma canker on peach. On vegetables we have seen bacterial stalk rot on sweet corn; bacterial wilt on cucumber; scab and southern stem blight on potato; early blight, bacterial canker, tomato spotted wilt virus, tomato mosaic virus, and Septoria leaf spot on tomato.
On herbaceous ornamentals we have diagnosed downy mildew on coreopsis; Fusarium stem rot on dianthus; blackleg (Pythium) on geranium; black root rot on petunia; bacterial soft rot on African violet; southern stem blight on portulaca; nutritional problems from high soil pH on chrysanthemum. On turf we have seen brown patch and necrotic ringspot. On woody ornamentals we have seen black root rot on holly and barberry; Phytophthora root rot on juniper; powdery mildew on dogwood; Dutch elm disease on elm; and Phyllosticta leaf spot on maple.

| UKREC-Princeton, KY, June 18 - 25 | |
| European Corn Borer | 0 |
| Corn Earworm | 0 |
| True Armyworm | 14 |
| Southwestern Corn Borer | 152 |
| Fall Armyworm | 0 |
| Lexington, KY, June 21 - 28 | |
| Black Cutworm | 0 |
| European Corn Borer | 1 |
| Corn Earworm | 8 |
| Diamondback Moth | 79 |
| Cabbage Looper | 6 |
| Beet Armyworm | 6 |
Lee Townsend
Extension Entomologist