
Carbohydrate Metabolism - Mono & Oligosaccharides, Glycosides, Ascorbic Acid & Other Sugar Derivatives

Carbohydrate Metabolism - Mono & Oligosaccharides, Glycosides, Ascorbic Acid & Other Sugar Derivatives
The purpose of today’s lecture is to examine the source of carbohydrates and cover carbohydrate chemistry of importance to plant metabolism.
READINGS
a) REQUIRED:
1- Chapter 13, pp 567-608, of the 2015 Biochemistry & Molecular Biology of Plants class text.
b) OPTIONAL:
1- Linster, C.L., T.A. Gomez, K.C. Christensen, L.N. Adler, B.D. Young, C. Brenner, and S.G. Clarke. 2007. Arabidopsis VTC2 Encodes a GDP-L-Galactose Phosphorylase, the Last Unknown Enzyme in the Smirnoff-Wheeler Pathway to Ascorbic Acid in Plants. J. Biol. Chem. 282:18879-18885.
2- Nägele, T., Henkel, S., Hörmiller, I., Sauter, T., Sawodny, O., Ederer, M., Heyer, A. G. 2010. Mathematical Modeling of the Central Carbohydrate Metabolism in Arabidopsis Reveals a Substantial Regulatory Influence of Vacuolar Invertase on Whole Plant Carbon Metabolism. Plant Physiology. 153: 260-272.
3- Lunn, J.E. 2002. Evolution of sucrose synthesis. Plant Physiology 128: 1490-1500.
4- Muller, J., R. A. Aeschbacher, A. Wingler, T. Boller and A. Wiemken. 2001. Trehalose and Trehalase in Arabidopsis. Plant Physiology 125: 1086-1093.
5- Black, M., F. Corbineau and D. Come. 1999. Water Content, Raffinose, and Dehydrins in the Induction Desiccation Tolerance in Immature Wheat Embryos. Plant Physiology 120: 463-471.
6- Wheeler, G.L., M.A. Jones and N. Smirnoff. 1998. The biosynthetic pathway of vitamin C in higher plants. Nature 393: 365-369.
Elemental Plants & people...
| Element | Zea mays |
Homo
sapiens (humans) |
| Hydrogen | 1705 | 2038 |
| Carbon | 1000 | 1000 |
| Oxygen | 765 | 252 |
| Nitrogen | ||
| Potassium | 6.5 | 6 |
| Calcium | 1.6 | 25 |
Phosphorus |
1.8 | 22 |
| Magnesium | 2.0 | 1.4 |
| Sulfur | 1.5 | 5.2 |
| Chlorine | 1.1 | 2.8 |
| Iron | 0.4 | 0.05 |
The overall equation for aerobic respiration is:
The overall equation for photosynthesis is the reverse:
Stochiometric presentation of Calvin-Benson cycle with all intermediate structures from Garrett and Grisham, 1995 text:
The elemental formula for a simple hexose sugar such as glucose is C6H12O6.
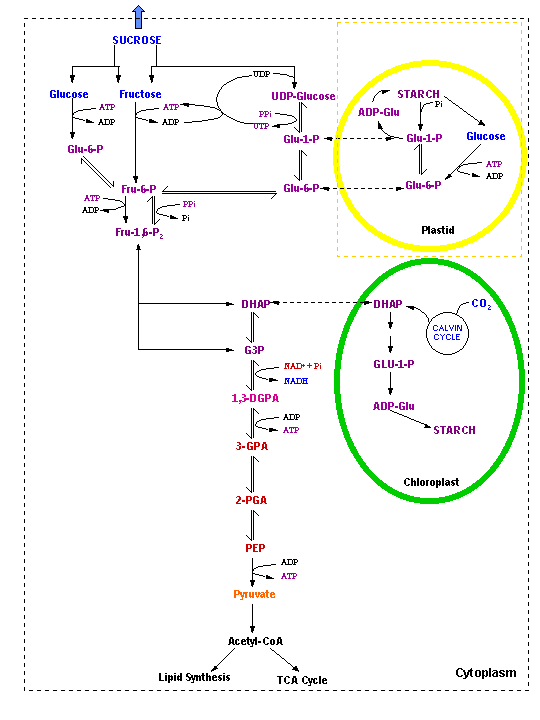
What is the metabolic fate of the glucose formed in photosynthesis?
Source Sink
Principal exports of photosynthetically active chloroplasts:
What is the form of reduced carbon exported from leaves and the form in which it is translocated to other plant parts?
A Brief Review of Carbohydrate Chemistry
Carbohydrates are polyhydroxy compounds (poly-alcohols) that contain a carbonyl (C=O) group of the general formula (CH2O)n. Like proteins and lipids, they can have structural, functional or storage roles. As is the case for proteins and nucleic acids they exist as monomers, oligomers and polymers. The monomers contain an aldehyde or ketone group, designated aldoses or ketoses. The simplest aldose is glyceraldehyde and the simplest ketose is dihydroxyacetone.
All the structures and background reading can be found in general biochemistry texts such as Garrett and Grisham 1995 text in the UK library, General Organic and Biochemistry, Chapter 17 -- Carbohydrates and Biochemistry Online: An Approach Based on Chemical Logic (chapter 3) by Dr. Henry Jakubowski.
As most of this is a review of general biochemistry, I will go through the general background and structures quickly until we get to important points I wish to make about certain plant carbohydrates.
Aldoses with at least 3 carbons and ketoses with at least 4 carbons contain chiral centers. The nomenclature for such molecules must specify the configuration about each asymmetric center and drawings of these molecules must be based on a system that clearly specifies these configurations. The Fischer projection system is a simple way to accomplish this.
Glyceraldehyde is chiral because its central carbon, C-2, has 4 different groups attached to it. A monosaccharide is designated D if the hydroxyl group on the highest numbered asymmetric carbon is drawn to the right in a Fischer projection. Note that the D and L stereoisomers are mirror images or enantiomers. Remember the D forms of monosaccharides predominate in nature. Longer aldoses and ketoses can be regarded as extensions of glyceraldehyde and dihydroxyacetone with chiral H-C-OH groups inserted between the carbonyl carbon and the primary alcohol group. The structures of important 4, 5 and 6 carbon aldoses, known as tetroses, pentoses and hexoses will be briefly overviewed in class.
Pairs of isomers that have opposite configurations at one or more of the chiral centers but that are not mirror images of each other are called diastereomers. Two sugars that differ in configuration at only one chiral center are described as epimers. For example, D-mannose and D-talose are epimers and D-glucose and D-mannose are epimers, whereas D-glucose and D-talose are not epimers but merely diastereomers. Sugars can exists as either configurational isomers (interconverted only by breaking covalent bonds) and conformational isomers. Configurational isomers can only be interconverted by breaking covalent bonds. See Biochemistry Online by Dr. Jakubowski (chapt. 3) for a nice review of sugar isomers.
The relationship of longer-chain ketoses to dihydroxyacetone is essentially the same as longer-chain aldoses are related to glyceraldehyde.
Fischer projections give a simplistic and not completely accurate depiction of the actual structure of most monosaccharides especially those with 5 or more carbons. A very interesting and important aspect of sugar structure is the ability to form cyclic structures with formation of an additional asymmetric center. Alcohols can readily react with aldehydes to form hemiacetals. Similarly ketones can react with alcohols to form hemiketals. This is illustrated in General Organic and Biochemistry, Chapter 17 -- Carbohydrates and Biochemistry Online: An Approach Based on Chemical Logic (chapt. 3).
Sir Norman Haworth showed that a linear form of an aldohexose can undergo such an intramolecular reaction to form a cyclic hemiacetal. The resulting 6-C oxygen containing ring structure is reminiscent of pyran and is called a pyranose. A corresponding intramolecular reaction of a ketohexose gives a cyclic hemiketal. The 5-C ring is similar to furan and is designated a furanose.
As hemiacetals and hemiketals are formed, the carbon atom that carried the carbonyl function becomes attached to 2 oxygen atoms and becomes an asymmetric carbon atom (and also the most oxidized). This carbon is known as the anomeric carbon and sugars that differ only in their configuration about this carbon atom are called anomers. The 2 anomers which can be formed upon cyclization are designated
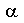 (OH down) and ß
(OH up). The Haworth projections of D-glucose
and of D-ribose are:
(OH down) and ß
(OH up). The Haworth projections of D-glucose
and of D-ribose are:
Haworth projections are simple and give an illustration of the ring structure of monosaccharides, but they do not take into account the 3D structure of sugars. Sugar molecules are not planar as might be implied by Fischer or Haworth projections. Instead they have puckered conformations of the rings, two favored structures of which are the chair and boat conformations.
The chair conformations are more stable than the boat conformations and such ring structures are more stable when bulky substituents (e.g. CH2OH) occupy equatorial rather than axial positions.
There are many derivatives of mono- and oligosaccharides that are of significance in plant biochemistry. Only the most important ones will be mentioned here.
Sugar Esters
The major sugar esters are sugar phosphates. Phosphorylated derivatives include intermediates of the Calvin-Benson cycle, biosynthetic precursors to other carbohydrates, metabolic intermediates of sugars used as fuel and a major constituent of RNA.
Deoxy Sugars
Deoxy sugars as the name implies are monosaccharides in which H replaces
one or more hydroxyl groups. 2-Deoxy-D-ribose is a major part
of DNA. 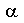 -L-Fucose (6-deoxy-L-galactose) is a component of some cell walls.
-L-Fucose (6-deoxy-L-galactose) is a component of some cell walls.
Amino Sugars
Amino sugars such as D-glucosamine and D-galactosamine have an amino group instead of a hydroxyl group at the C-2 position. They are found in many oligo- and polysaccharides including chitin, a polysaccharide found in the exoskeletons of arthropods.
Sugar Alcohols
The reduction of the carbonyl group of aldoses and ketoses results in a sugar alcohol or alditol (designated by addition of -itol to the parent sugar name). Sugar alcohols are usually linear molecules unable to cyclize in the manner of aldoses. However like unmodified sugars, sugar alcohols such as sorbitol, mannitol and xylitol are sweet tasting and widely used to sweeten sugarless gums and mints. Ribitol is a constituent of flavin coenzymes. Glycerol and the cyclic poly-alcohol, myo-inositol, are important components of lipids. e.g.:
Sugar Acids
Sugars with free anomeric carbon atoms (the carbonyl C) are reasonably good reducing agents being fairly readily oxidized by certain oxidants such as H2O2, ferricyanide, some metals such as Cu+2, etc. Such a reaction converts the aldose to an aldonic acid such as D-gluconic acid formed by oxidation of the aldehyde of D-glucose producing a carboxylic acid at C-1. For example, addition of alkaline CuSO4 results in a reduced product, cuprous oxide (Cu2O) which forms a red precipitate.
Carbohydrates that can reduce oxidizing agents in this way are known as reducing sugars.
What happens to sugars that reduce such compounds?
Glucose can be oxidized at C-6; or C-1 and C-6 yielding a dicarboxylic acid. Oxidation of glucose at C-6 gives D-glucuronic acid, which can be metabolized to the further sugar derivative, L-ascorbic acid or vitamin C in animals that can synthesize ascorbic acid.
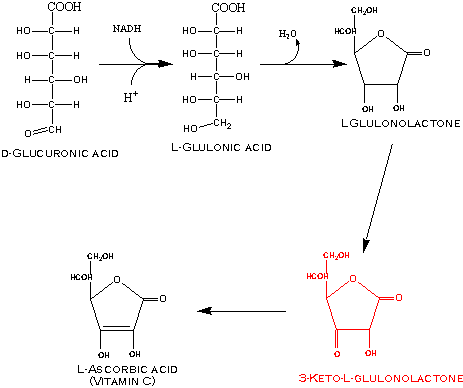
Synthesis of ascorbic acid from D-glucuronic acid involves a reduction of the aldehyde of D-glucuronic acid yielding L-gulonic acid that is dehydrated and cyclized to L-gulonolactone by a lactonase. The L-gulonolactone is oxidized by L-gulonoxidase to 3-keto-L-gulonolactone, which rearranges to ascorbic acid.
Can humans synthesize L-ascorbic acid?
Why do plants make this molecule?
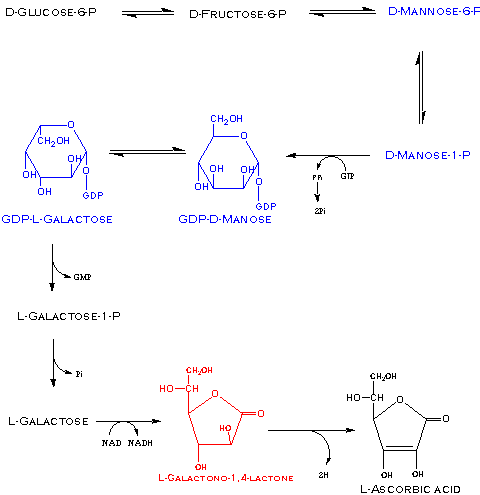
Although ascorbic acid is very important in plant metabolism and is found at high concentrations in many plant tissues (e.g. 1-5 mM in leaves and 25 mM in chloroplasts) and plants are the main source of vitamin C in the human diet, the biosynthetic pathway for this sugar acid was not resolved in plants until 1998 and all genes encoding the enzymes until 2007. It had been known for some time that plants can readily synthesize ascorbic acid from L-galactono-1,4-lactone via mitochondrial L-galactono-1,4-lactone dehydrogenase. However, it was previously thought that the precursor to L-galactono-1,4-lactone in plants was D-galacturonic acid. Synthesis of L-galactono-1,4-lactone from D-galacturonic acid would require inversion of the hexose carbon skeleton which has been demonstrated not to occur in plants from 14C-glucose. The key to this puzzle appears to be solved by Wheeler et al. (1998) who showed that 14C-glucose is converted to ascorbic acid via D-mannose and L-galactose intermediates rather than D-galactose. D-mannose and L-galactose are interconverted by a GDP-D-mannose-3,5-epimerase and both of these monosaccharides are efficient precursors of ascorbic acid in plants. The Smirnoff-Wheeler pathway for L-ascorbic biosynthesis in plants is given in Figures 4 of Wheeler et al. (1998) and Linster et al. (2007):
Ascorbic acid is very readily oxidized making it a powerful and most important water-soluble reducing agent (antioxidant) in biological systems especially chloroplasts. Loss of one e- yields L-ascorbate free radical (semidehydro-L-ascorbate) and loss of two e- results in dehydro-L-ascorbic acid:
Dehydro-L-ascorbic acid can be reduced back to ascorbic acid by the glutathione/glutathione reductase system which will be discussed in Lecture 25 under sulfur metabolism.
Glycosides/Oligosaccharides
Sugars in the form of the cyclic hemiacetals and hemiketals (see Lecture 5) can react (condense) with an alcohol, amine or a thiol forming an acetal or ketal. The bond formed by this dehydration reaction of the anomeric carbon of a sugar with any of these groups is known as a glycosidic linkage or glycoside. Di-, oligo- and polysaccharides are formed when glycosidic bonds are made between an anomeric carbon of one sugar and hydroxyl group(s) of additional sugar(s).
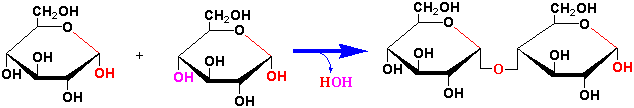
Important disaccharides in plants include sucrose, maltose, trehalose and cellobiose (see Chapter 17):
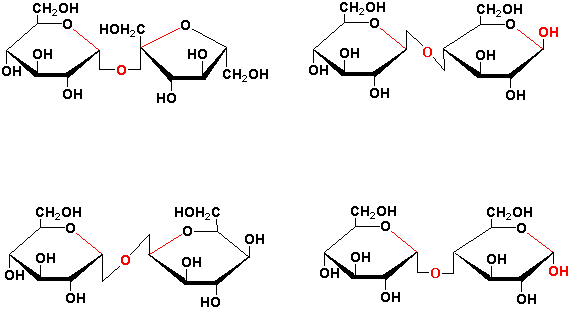
In the naming of sugar polymers, the monosaccharide residues involved, the position of the linked carbons atoms and the configuration of the glycosidic bond must be given. Thus sucrose, maltose, trehalose and cellobiose are:
Sugars & food/nutrition
1. What is rebaudioside A (give the structure)?
2. What the basis of the interest in rebaudioside A?
3. How is rebaudioside A synthesized?
Sucralose
What are the properties of sucrose that makes it highly suited for its very extensive role in transport and storage of reduced carbon skeletons?
The end of the carbohydrate containing the free anomeric carbon is called the reducing end. With sucrose both of the anomeric carbon atoms are substituted -- neither has a free hydroxyl group. The bound anomeric carbons cannot be converted into the aldehyde or ketone configurations and cannot participate in oxidation/reduction reactions. Therefore sucrose is not a reducing sugar. Which of the above mentioned plant disaccharides are reducing sugars?
Other common molecules involving glycosidic linkages of the anomeric carbons of sugars with compounds other than additional sugars are nucleosides and some secondary compounds e.g. vanillin glucoside.
What plant care recommendations were made in the wake of the mare-reproductive-loss syndrome that hit central KY 8 years ago and what biochemical bases might these recommendations have had?
A very interesting group of glycosides made by certain plants are the cyanogenic glucosides. This group of compounds as well as some other glycosides is among plant's arsenal of chemical defenses. The cyanogenic glucosides are rather inert, benign molecules from which the highly toxic compound, cyanide, is released upon attack by an insect or disease-causing organism. An example of a cyanogenic glucoside is amygdalin found in many Roseaceae seeds such as bitter almonds, kernels of cherries, peaches and apricots:
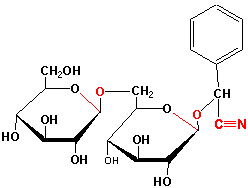
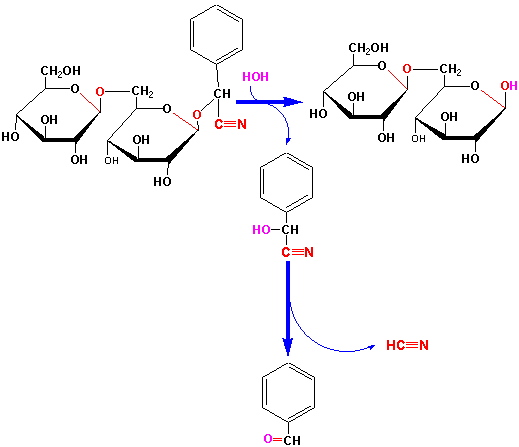
Amygdalin and other cyanogenic glucosides are hydrolyzed by a glucosidase (ß in this case) when this plant defense pathway is elicited leading to release of hydrogen cyanide:
Important glycosides include glycoproteins that have numerous structural and functional roles in plants:
Many important antibiotics are oligosaccharides or contain oligosaccharide groups.
Sucrose is the most important plant disaccharide being the principle form in which photosynthetic energy is transported throughout plant tissues from the sucrose source (photosynthetic leaves) to non-photosynthetic tissues (sinks). Two enzymes are known which are capable of sucrose synthesis in plants, sucrose synthase and sucrose phosphate synthase:
Most if not all sucrose in nature is synthesized by sucrose phosphate synthase and sucrose synthase is thought to be mainly involved in sucrose catabolism.
Wang, L., Lu, Q., Wen, X., and Lu, C. (2015). Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiology.
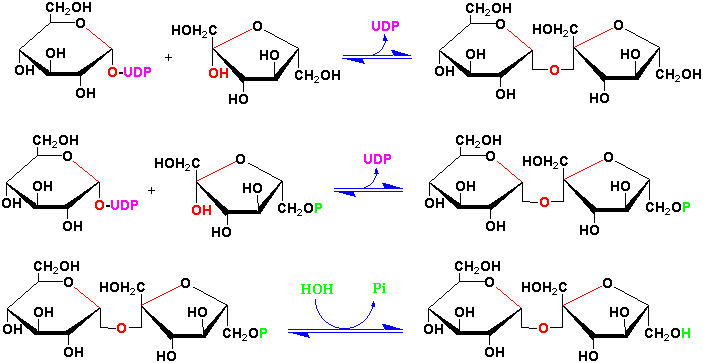
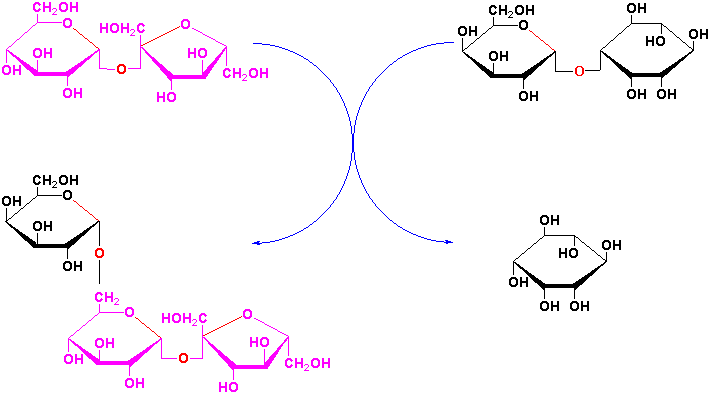
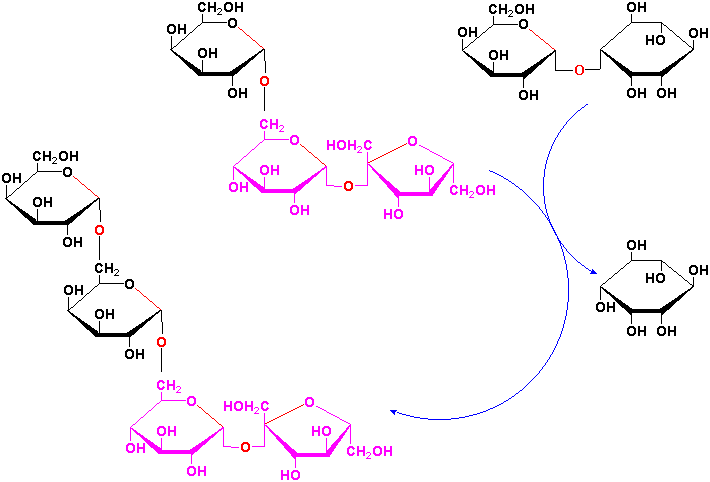
Sucrose is a precursor to a group of carbohydrates in plants known as the raffinose family of oligosaccharides found in many plant seeds especially legumes. This family contains the trisaccharide raffinose, the tetrasaccharide stachyose and the pentasaccharide verbascose:
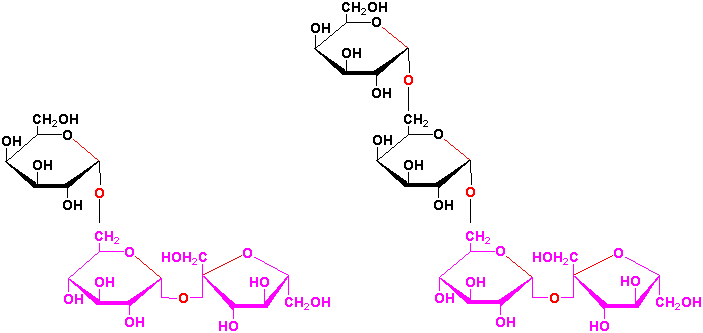
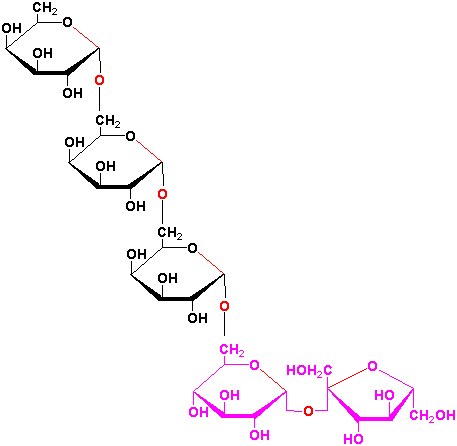
These oligosaccharides are biosynthesized by the successive transfer of D-galactosyl residues from galactinol (galactose-
 -1,1-myoinositol) to
the C-6 hydroxyl group of the terminal D-glucosyl of D-galactosyl residue of the
preceeding oligosaccharide of the sequence:
-1,1-myoinositol) to
the C-6 hydroxyl group of the terminal D-glucosyl of D-galactosyl residue of the
preceeding oligosaccharide of the sequence:
etc.
Recently anti-flatulence products (e.g. Beano®) have been on the market. How do you think these might function?
Galactinol is synthesized by the formation of a glycoside between UDP-D-galactose and myoinsitol by UDP-D-glactose:myoinositol galactosyltransferase:
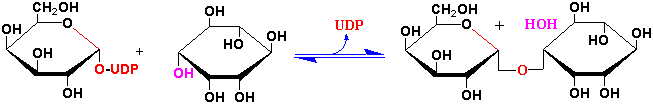
What is phytate and why is it important in agriculture (see section 19.10.11 & Fig. 19.49 of the class text)?
Another important plant disaccharide, trehalose, is synthesized from UDP-D-glucose + D-glucose-6-phosphate by trehalose phosphate synthase and trehalose phosphatase:
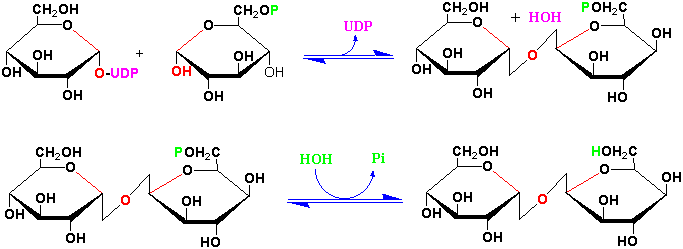
Reading Assignments for the second carbohydrate discussions:
a) REQUIRED:
1- Stoop, J.M., J. Van Arkel, J.C. Hakkert, C. Tyree, P.G. Caimi, and A.J. Koops. 2007. Developmental modulation of inulin accumulation in storage organs of transgenic maize and transgenic potato. Plant Science 173:172-181.
b) OPTIONAL:
1- Tamura, K.-i., Y. Sanada, K. Tase, A. Kawakami, M. Yoshida and T. Yamada. 2014. Comparative study of transgenic Brachypodium distachyon expressing sucrose:fructan 6-fructosyltransferases from wheat and timothy grass with different enzymatic properties. Planta: 1-10.- http://link.springer.com/article/10.1007/s00425-013-2016-8#
2- Smith, A. 1999. Making Starch. Curr. Opinion in Plant Biology 2:223-229.
3- Bush, D. 1999. Sugar transporters in plant biology. Curr. Opinion in Plant Biology 2:187-191.
4- Crumpton-Taylor, M., Grandison, S., Png, K. M. Y., Bushby, A. J., Smith, A. M., 2012. Control of Starch Granule Numbers in Arabidopsis Chloroplasts. Plant Physiology. 158, 905-916.
5- Vijin, I. and Smeekens, S. 1999. Fructan: More Than a Reserve Carbohydrate? Plant Physiology 120: 351-359.
6- Andriotis, V.M.E., Pike, M.J., Schwarz, S.L., Rawsthorne, S., Wang, T.L., and Smith, A.M. (2012). Altered Starch Turnover in the Maternal Plant Has Major Effects on Arabidopsis Fruit Growth and Seed Composition. Plant Physiology 160, 1175-1186.
| All materials © 2018 David Hildebrand, unless otherwise noted. | |||||||
| home | syllabus | lecture schedule & web notes | virtual office hours | messages & answers from the instructor | supplementary material | related links | What's new? |