
Lecture Seventeen B
Carbohydrate Metabolism -- Polysaccharides, Starch Synthesis and Turnover, Fructans

The purpose of today’s lecture is to discuss the biosynthesis and metabolism of oligo- and polysaccharides, particularly sucrose and starch, and the interaction of key points of carbohydrate metabolism with other major metabolic pathways in plants.
Reading Assignments for 2nd Carb Discussions:
a) REQUIRED:
1- Fukutomi, D., Yoshinaka, K., Kawamoto, S., Mitsunari, T., Kajita, S., Kawai, S., 2013. High-level fructooligosaccharide production in transgenic tobacco plants. Plant Biotechnology. 30, 77-81.
b) OPTIONAL:
1- Tamura, K.-i., Y. Sanada, K. Tase, A. Kawakami, M. Yoshida and T. Yamada. 2014. Comparative study of transgenic Brachypodium distachyon expressing sucrose:fructan 6-fructosyltransferases from wheat and timothy grass with different enzymatic properties. Planta: 1-10.- http://link.springer.com/article/10.1007/s00425-013-2016-8#
2- Smith, A. 1999. Making Starch. Curr. Opinion in Plant Biology 2:223-229.
3- Wang, L., Lu, Q., Wen, X., and Lu, C. (2015). Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiology.
4- Crumpton-Taylor, M., Grandison, S., Png, K. M. Y., Bushby, A. J., Smith, A. M., 2012. Control of Starch Granule Numbers in Arabidopsis Chloroplasts. Plant Physiology. 158, 905-916.
5- van Arkel, J., Vergauwen, R., Sévenier, R., Hakkert, J. C., van Laere, A., Bouwmeester, H. J., Koops, A. J., van der Meer, I. M., 2012. Sink filling, inulin metabolizing enzymes and carbohydrate status in field grown chicory (Cichorium intybus L.). J. Plant Physiol. 169, 1520-1529.
6- Andriotis, V.M.E., Pike, M.J., Schwarz, S.L., Rawsthorne, S., Wang, T.L., and Smith, A.M. (2012). Altered Starch Turnover in the Maternal Plant Has Major Effects on Arabidopsis Fruit Growth and Seed Composition. Plant Physiology 160, 1175-1186.
7- Cakir, B., Shiraishi, S., Tuncel, A., Matsusaka, H., Satoh, R., Singh, S., Crofts, N., Hosaka, Y., Fujita, N., Hwang, S.-K., et al. (2016). Analysis of the rice ADPglucose transporter (OsBT1) indicates the presence of regulatory processes in the amyloplast stroma that control ADPglucose flux into starch. Plant Physiology.
The structures and additional background reading can be found in general biochemistry texts such as the Garrett and Grisham 1995 text (available in the library), Chapt. 13 of the class text and General Organic and Biochemistry, Chapter 17 -- Carbohydrates.
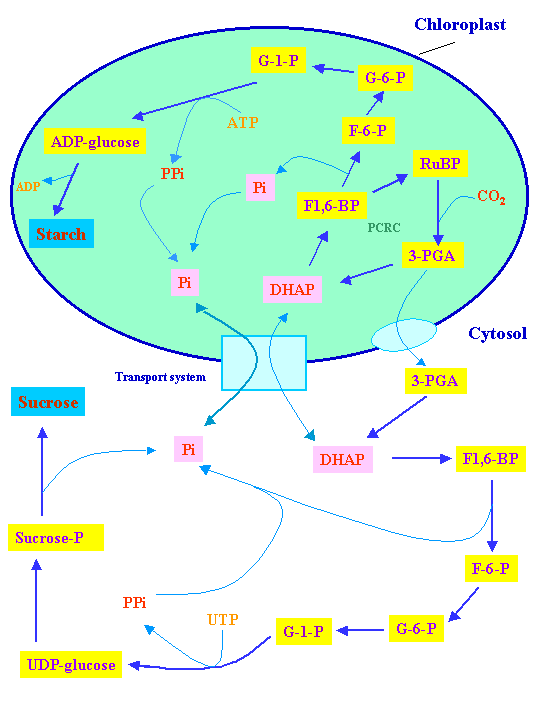
Utilization of triose phosphate generated by photosynthesis in starch, sucrose and/or fructan synthesis is tightly controlled (see Chapt. 13 of class text). Since 5/6 of triose phosphate synthesized in photosynthesis is needed for regeneration of RuBP, a maximum of 1/6 of triose phosphate formed is available for synthesis of fructose and other molecules in the cytoplasm. In C3 plants only ~ 1/8 of triose phosphate is available for export from chloroplasts due to photorespiration. If more than 1/6 to 1/8 of the triose phosphate were removed from the Calvin-Benson cycle, RuBP could no longer be regenerated and the cycle would collapse.
However, CO2 fixation reactions of photosynthesis can only proceed if the triose phosphate formed is utilized in synthesis of molecules such as sucrose, starch, fructans or sink development. Thus formation of storage and transport carbohydrates can only but must proceed when sufficient photosynthesis occurs. Starch buildup in chloroplasts can only proceed so far after which photosynthesis shuts down. Thus for efficient plant growth photosynthesis must be coordinated with delivery of photosynthate to sinks. Some of the principal steps of carbohydrate metabolism were shown in lecture 17A:
As mentioned in the last lecture reduced carbon (photosynthate) is transported from source to sink tissues in the form of sucrose. Also as mentioned in the last lecture, sucrose is not made in plastids. The principal avenue for transport of reduced carbon out of chloroplasts has been thought to be in the form of the triose phosphates, DHAP and G3P. These triose phosphates can be utilized in respiration, in the synthesis of other molecules in plants such as lipids or for synthesis of sucrose. G3P and DHAP can be condensed by an aldolase to form Fru-1,6-P2 in the cytoplasm as in the chloroplasts. Fru-1,6-P2 can be converted into Fru-6-P by PPi: fructose 6-P phosphotransferase. Fru-6-P can be isomerized to Glu-6-P which in turn can be converted into Glu-1-P by a mutase. Glu-6-P and Glu-1-P are the main forms in which photosynthate is delivered to plastids of sink tissues for accumulation of starch.
As mentioned in Lecture 11, sucrose can be synthesized from hexose monophosphates by sucrose synthase or sucrose phosphate synthase. Both enzymes are freely reversible in vitro, but in vivo sucrose phosphate synthase is associated with sucrose phosphatase which catalyzes an irreversible reaction rendering the coupled reaction only toward sucrose synthesis.
In the case of sucrose synthase the in vivo sucrose concentrations are nearly always much higher than fructose or UDP-glucose resulting in this reaction essentially always going in the direction of sucrose cleavage. Invertases on the other hand only catalyze the cleavage of sucrose into glucose and fructose.
Sucrose transport to different plant parts is not a passive process and is instead modulated by a variety of sucrose and hexose transporters that fulfill a variety of essential roles in partitioning of assimilate between source and sink tissues. In the case of sucrose loading into phloem tissue, symport is driven by a proton motive force that is generated by a proton-ATPase as in chloroplasts and mitochondria but consuming rather than generating ATP. There is evidence for at least six sucrose symporter genes in Arabidopsis (Fig. 1 Bush 1999).Wang et al. (2015), Plant Physiology.
In some plant sink tissues sucrose is the principal form of reduced carbon storage and sucrose can build up to high levels in vacuoles of such storage tissues. Extracellular invertases are a major route for metabolism of sucrose and this is influenced by a number of signals and biotic or abiotic stresses (Fig. 2 Roitsch, 1999). Isozymes of invertase can be found in the cytosol and vacuoles in addition to the apoplast (Sturm, 1999). Further metabolism of hexoses generated by invertase or sucrose synthase requires their conversion back into hexose phosphates by hexokinases. Hexose phosphates are key intermediates in metabolism of carbohydrates:
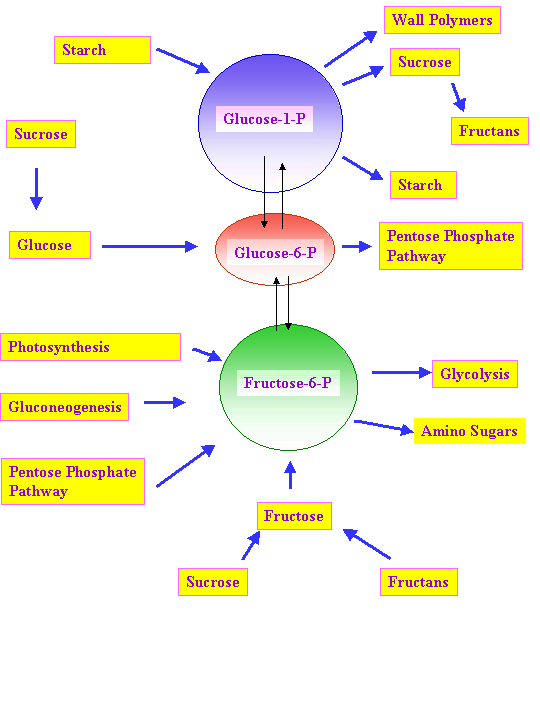
At least in rice the main import into amyloplasts for starch synthesis is ADP-glucose (Cakir et al., 2016).
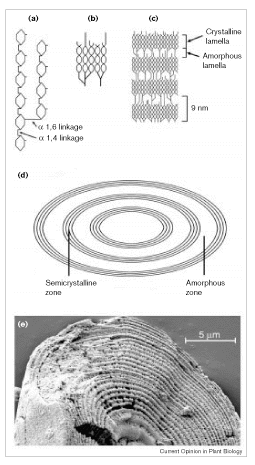
As mentioned previously the energy is stored in plants (and animals) as polysaccharides or triglycerides. The principal storage carbohydrate in plants is the polysaccharide, starch. Starch is composed of 2 polymers, amylose, amylopectin and in some cases phytoglycogen. Natural starch is usually ~10-30% amylose and 70-90% amylopectin. US cornstarch is normally ~25% amylose and 75% amylopectin. Amylose molecules are linear chains of D-glucose in
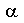 -1,4 linkages with molecular weights of several
thousand to 500,000. Amylopectin is a highly branched chain of
glucose monomers (amylose often has a few branches, 1-5, usually near the
reducing end of the molecule). Most of the glucose linkages in
amylopectin are
-1,4 linkages with molecular weights of several
thousand to 500,000. Amylopectin is a highly branched chain of
glucose monomers (amylose often has a few branches, 1-5, usually near the
reducing end of the molecule). Most of the glucose linkages in
amylopectin are 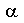 -1,4 as for amylose, whereas the branches are
-1,4 as for amylose, whereas the branches are 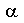 -1,6. Branches
occur ~every 12- 30 residues. Amylopectin molecular weights range up
to 100 x 106 with a typical molecule being ~200-400 nm
long. Some starch (e.g., from potato) is phosphorylated with up to 1
Pi per 300 glucose residues. Structures of amylose and amylopectin
are given in Figs. 1 and 2 of Smith 1999:
-1,6. Branches
occur ~every 12- 30 residues. Amylopectin molecular weights range up
to 100 x 106 with a typical molecule being ~200-400 nm
long. Some starch (e.g., from potato) is phosphorylated with up to 1
Pi per 300 glucose residues. Structures of amylose and amylopectin
are given in Figs. 1 and 2 of Smith 1999:
 2
2 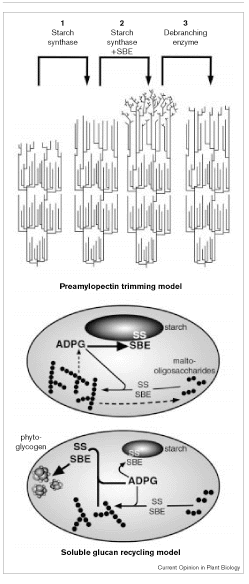
Zeeman S.C., Kossmann J., Smith A.M. (2010) Starch: Its Metabolism, Evolution, and Biotechnological Modification in Plants. Annual Review of Plant Biology 61:209-234. Fig. 1
Starch is synthesized and stored in plastids; chloroplasts in leaves and specialized organelles in storage tissues termed amyloplasts. The starch is densly packed in structures known as starch granules, often as starch crystals. Starch granules, which contain 0.1-1% lipid and 0.05-0.5% protein, vary in size from < 1 µm to > 100 µm. Starch granule structure and synthesis is shown in Fig. 1 of Smith (1999):
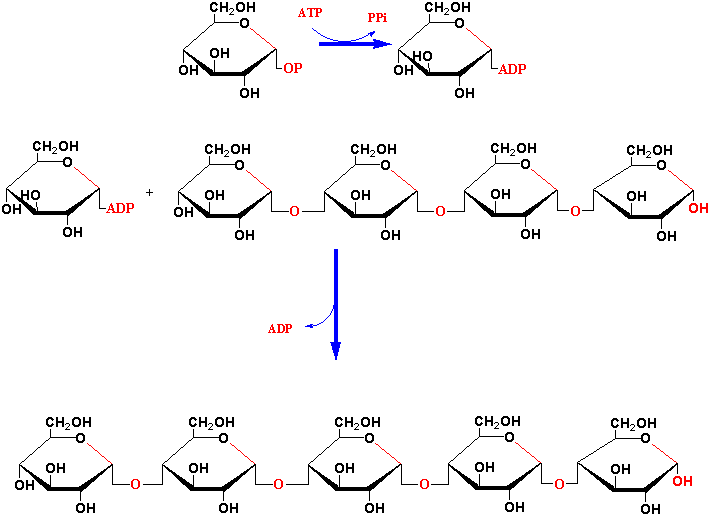
Both amylose and amylopectin synthesis begins with synthesis of ADP-glucose from glucose-1-phosphate and ATP by ADP-glucose pyrophosphorylase with liberation of pyrophosphate (see Fig. 2 Smith 1999). Next starch synthase catalyzes the formation of an
 -1,4 linkage
between the non-reducing end of a preexisting glucose chain and the
glucosyl moiety of ADP-glucose with release of ADP.
-1,4 linkage
between the non-reducing end of a preexisting glucose chain and the
glucosyl moiety of ADP-glucose with release of ADP.
Finally, the
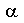 -1,6 linkages of
the branches are synthesized by starch branching enzyme that
hydrolyzes an
-1,6 linkages of
the branches are synthesized by starch branching enzyme that
hydrolyzes an 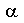 -1,4 linkage within the chain and then forms an
-1,4 linkage within the chain and then forms an 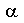 -1,6 linkage between
the reducing end of the spliced glucan chain and another glucose
residue.
-1,6 linkage between
the reducing end of the spliced glucan chain and another glucose
residue.
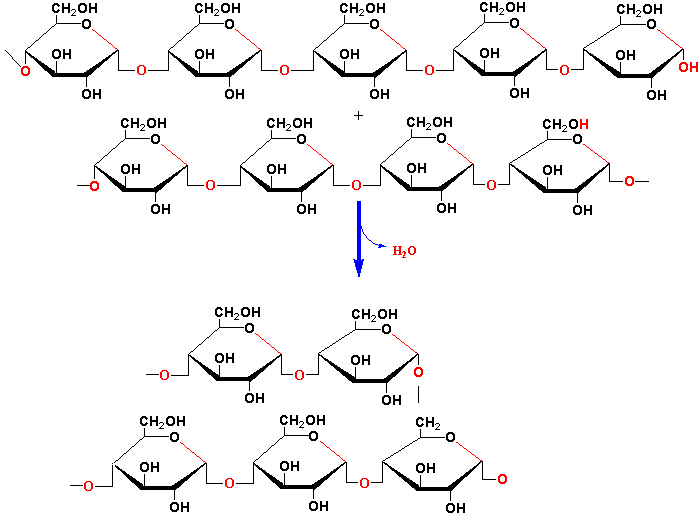
These branches have an average length of ~20 glucan units.
Starch is catabolized by 5 enzymes: α-amylase, β-amylase, α-glucosidase, starch phosphorylase, and α-dextrin 6-glucanohydrolase (debranching enzyme) (see Chapt. 13 of class text):
What is nature's most abundant polymer?
Fructans
Fructans are probably the most abundant storage carbohydrate in plants next to starch and sucrose. Fructans are linear or branched polymers of mostly ß-fructosyl-fructose linkages. Unlike sucrose they are synthesized and stored in vacuoles and can accumulate in the stems, bulbs and tubers of a number of plants (see Vijn and Smeekens, 1999 & Lattanzi et al., 2012 for details). Fructans are the main long-term storage molecules in the vegetative parts of many important economic plant species, including wheat, barley, rye, oat, and C3 forage grasses including tall fescue. The basic structure of a fructan is a trisaccharide, known as a kestose , which has a sucrose molecule linked to one additional fructose:
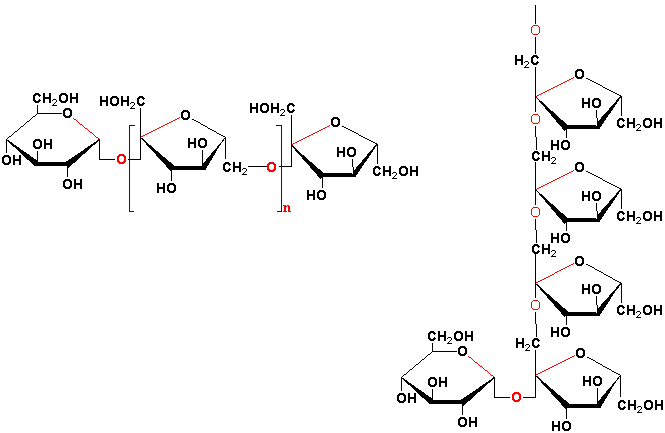
They are found as oligosaccharides of 5-10 fructose molecules in the neokestose type, ~50 residues in the inulin or 1-kestose group and ~200 for the levan or 6-kestose type. The inulin group has the fructose residue of sucrose in position 1 glycosidically linked with another fructose in the 2ß position. The levan type has the fructose residue of sucrose in position 6 glycosidically linked with another fructose in the 2ß position. Branched fructans found in grasses, known as graminanes, have fructose molecules bound by both 1-ß2 and 6-ß2 glycosidic linkages.
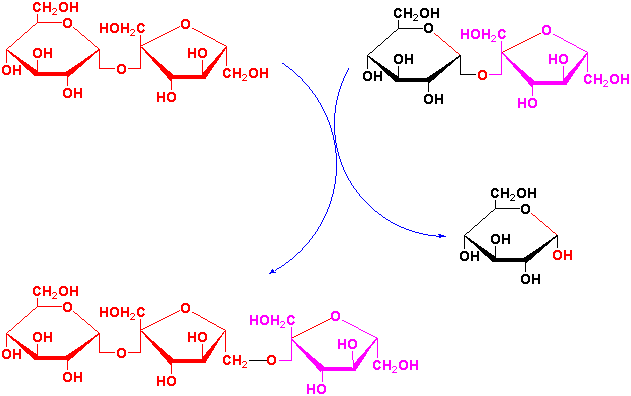
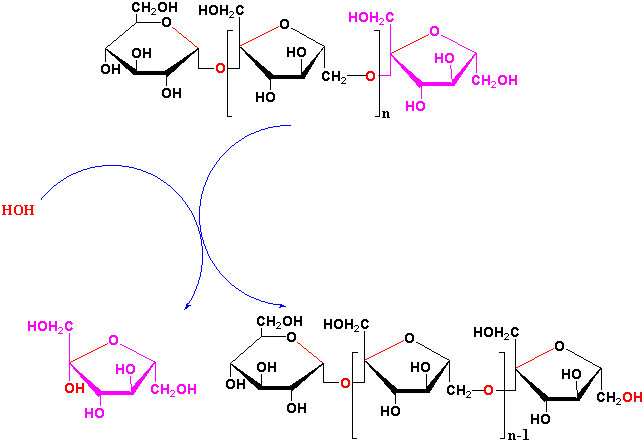
Fructan synthesis occurs in vacuoles and sucrose is the precursor although fructose only fructans are known. In the first step of fructan synthesis, the fructose moiety of a sucrose molecule is transferred to a second sucrose molecule by sucrose-sucrose fructosyl transferase forming a kestose + glucose:
Fructose groups are preferentially transferred from a trisaccharide kestose to longer kestoses rather than from sucrose by fructan-fructan fructosyl transferases (see Fig. 4 of Vijn and Smeekens, 1999).
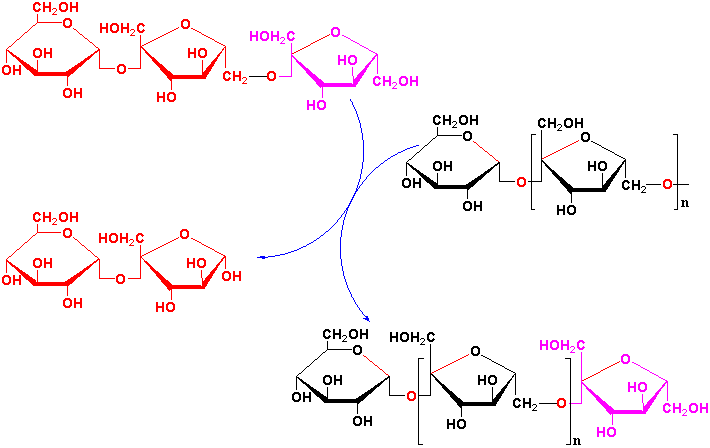
![[model of fructan synthesis]](model.gif)
A main current source of fructan is chicory (Cichorium intybus) van Arkel et al. (2012). Progress is bein made in genetic engineering productive major crop plants for more economical fructan production (Fukutomi et al., 2013).
Fructan exohydrolases degrade fructan polymers by cleaving terminal fructose residues.
What is thought to be the physiological function of fructans in plants?
How is the fructose made in plants?
Energy! ........... US Energy Supplies; World; CH3CH2OH
Reading Assignment for the last Carbohydrate Discussion for Class (to be updated):
a) REQUIRED:
1- Chapter 2, pp 45-110, of the Biochemistry & Molecular Biology of Plants class text.
2- Mutwil, M., S. Debolt, and S. Persson. 2008. Cellulose synthesis: a complex complex. Current Opinion in Plant Biology 11:252-257.
3- Mohnen, D. 2008. Pectin structure and biosynthesis. Current Opinion in Plant Biology 11:266-277.
b) OPTIONAL:
1- Sticklen, M.B. 2008. Plant genetic engineering for biofuel production: towards affordable cellulosic ethanol. Nat Rev Genet 9:433-443.
2- Himmel, M.E., S.-Y. Ding, D.K. Johnson, W.S. Adney, M.R. Nimlos, J.W. Brady, and T.D. Foust. 2007. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 315:804-807.
3- Sterling, H., J.D., M.A. Atmodjo, S.E. Inwood, V.S. Kumar Kolli, H.F. Quigley, M.G. Hahn, and D. Mohnen. 2006. From the Cover: Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. PNAS 103:5236-5241.
4- Bosca, S., C.J. Barton, N.G. Taylor, P. Ryden, L. Neumetzler, M. Pauly, K. Roberts, and G.J. Seifert. 2006. Interactions between MUR10/CesA7-Dependent Secondary Cellulose Biosynthesis and Primary Cell Wall Structure. Plant Physiol. 142:1353-1363.
5- Ding, S.Y., and M.E. Himmel. 2006. The Maize Primary Cell Wall Microfibril: A New Model Derived from Direct Visualization. J. Agric. Food Chem. 54:597-606.
6- Delmer and Amor. 1995. Cellulose biosynthesis. The Plant Cell 7:987-1000.
7- Yi, H., and Puri, V.M. (2012). Architecture-Based Multiscale Computational Modeling of Plant Cell Wall Mechanics to Examine the Hydrogen-Bonding Hypothesis of the Cell Wall Network Structure Model. Plant Physiology 160, 1281-1292.
| All materials © 2018 David Hildebrand, unless otherwise noted. | |||||||
| home | syllabus | lecture schedule & web notes | virtual office hours | messages & answers from the instructor | supplementary material | related links | What's new? |