
Lecture Nineteen
Lipids -- Introduction, Synthesis of Fatty Acids, Waxes and Cutin

Purpose and Outline
The purpose of today's lecture/discussion is introduce some aspects of the biological chemistry of plant glycerolipids you likely didn't study previously. We will also review saturated fatty acid biosynthesis with emphasis on aspects unique to plants. Finally we will briefly cover the synthesis of waxes and cutin in plants.
Reading Assignment for the First Lipids Discussion for Class:
a) REQUIRED:
1- Fich, E.A., Segerson, N.A., and Rose, J.K.C. (2016). The Plant Polyester Cutin: Biosynthesis, Structure, and Biological Roles. In Annual Review of Plant Biology, Vol 67, S.S. Merchant, ed., pp. 207-233..
2- Chapter 8 sect. 8.1 - 8.4, pp 337 -352 & sect. 8.9 pp 382 - 389 (Chapt 10 sect. 10.1 - 10.4, pp 456-476 old text) of the Biochemistry & Molecular Biology of Plants class text.
b) OPTIONAL:
1- Yeats, T. H. and J. K. C. Rose. 2013. The Formation and Function of Plant Cuticles. Plant Physiology 163: 5-20.
2- Jetter, R., and Riederer, M. (2016). Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiology 170: 921-34.
3- Maier, T., M. Leibundgut, and N. Ban. 2008. The Crystal Structure of a Mammalian Fatty Acid Synthase. Science 321:1315-1322.
4- Samuels, L., L. Kunst, and R. Jetter. (2008). Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annual Review of Plant Biology 59:683-707.
5- Focks, N. and C. Benning. (1998). wrinkled 1: A novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118:91-101.
In biology the term lipid can refer to almost any molecule hydrophobic enough such that it would partition into an organic solvent from an aqueous solution. Major lipids in plants include the glycerolipids and other fatty acid derivatives, isoprenoids and some phenylpropanoids. In the narrow sense lipids only include glycerolipids. In the next 3 lectures we will discuss the metabolism of glycerolipids and some other important fatty acid derivatives.
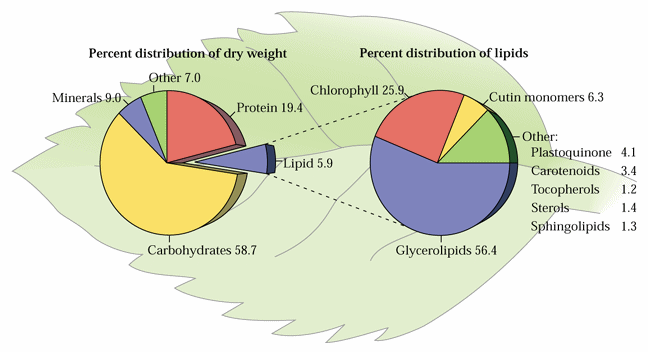
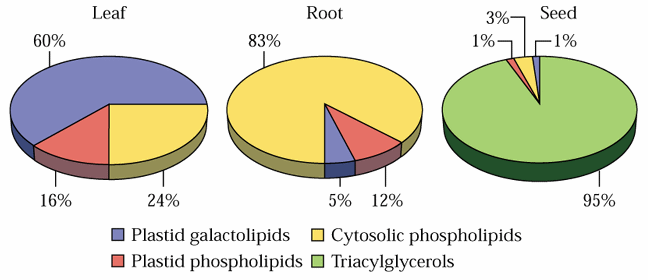
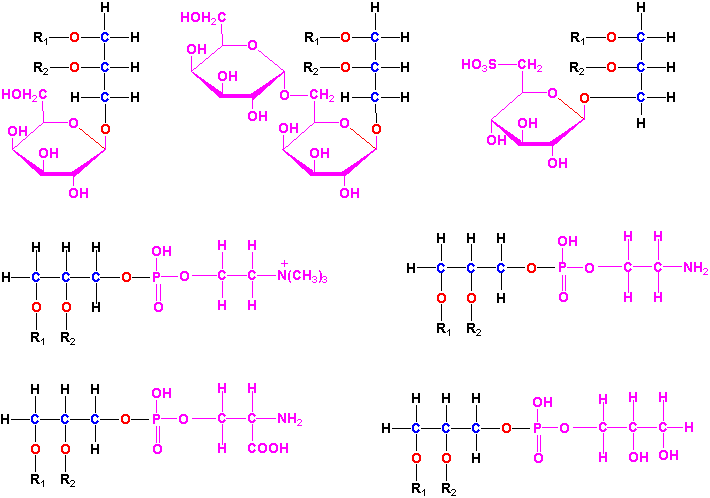
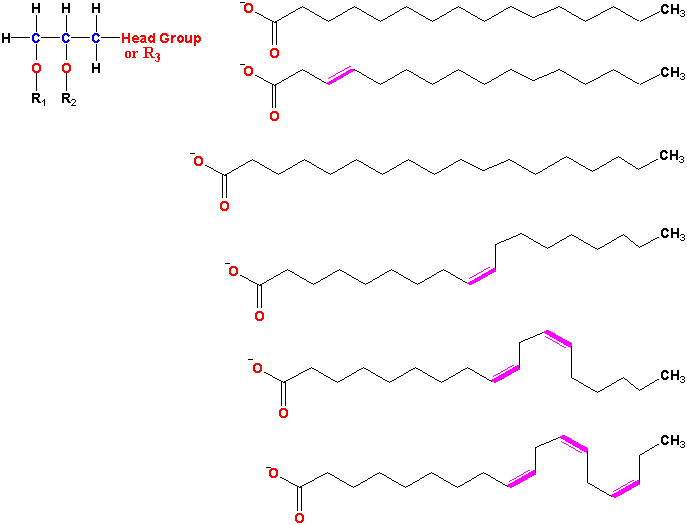
Glycerolipids are molecules in which in glycerol is esterified to 1-3 fatty acids. Monoglycerides, having a fatty acid only esterified to the sn-1, or -2 position of glycerol, are rare in biological systems. Triglycerides (TG) are the principal storage lipids and diglycerides are the main membrane lipids. Most diglycerides have polar head groups including either a phosphate group for the phospholipids (PL) or a sugar or sugar derivative for the glycolipids (GL). The main phospholipids in plants are phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS) and cardiolipin (CL). The principal glycolipids are monogalactosyldiacylglycerol (MGD), digalactosyldiacylglycerol (DGD) and sulfoquinovosyldiacylglycerol (SL). These are illustrated in Fig. 1 of Ohlrogge and Browse (1995) The Plant Cell 7, 957-970 and Tables 10.2 & 10.3 and Figure 10.5 of the class text.
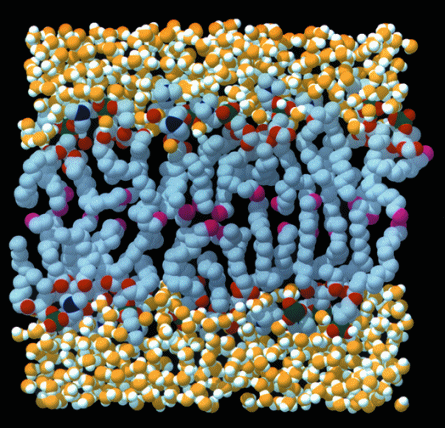
Plant cell diagram
Fatty acids (FAs) are organic acids of the general formula RCOOH where R is an alkyl or alkane group -- pure hydrocarbon or a substituted hydrocarbon. Thus FAs are alkyl acids = CH3(CH2)nCOOH. Such alkyl acids are not sufficiently hydrophobic to generally be considered fatty acids unless n>4. Some short and medium chain fatty acids and related acids with chemical names and common names where available are given in the following table:
Table 1. Short and medium chain fatty acids.
Formula |
(chemical name) |
# Carbons |
HCOOH |
formic acid |
1 |
CH3COOH |
acetic acid |
2 |
CH3CH2COOH |
proprionic |
3 |
CH3(CH2)2COOH |
butyric |
4 |
CH3(CH2)3COOH |
(pentanoic) |
5 |
CH3(CH2)4COOH |
(hexanoic) |
6 |
CH3(CH2)5COOH |
heptanoic |
7 |
CH3(CH2)6COOH |
(octanoic) |
8 |
CH3(CH2)7COOH |
nonanoic |
9 |
CH3(CH2)8COOH |
(decanoic) |
10 |
CH3(CH2)9COOH |
undecanoic |
11 |
CH3(CH2)10COOH |
(____________) |
12 |
CH3(CH2)11COOH |
tridecanoic |
13 |
CH3(CH2)12COOH |
(____________) |
14 |
CH3(CH2)13COOH |
pentadecanoic |
15 |
According to standard chemical nomenclature, the double bonds of unsaturated fatty acids are numbered from the carboxylic acid end of the molecule. Thus linolenic acid is actually cis, cis, cis D9, D12, D15-octadecatrienoic acid.
Table 2. Important long-chain fatty acids:
| (hexadecanoic) palmitic 16:0  |
(cis 7-hexadecenoic)
[palmitoleic(D9)] 16:1  |
7,10-hexadecadienoic 16:2  |
7,10,13-hexadecatrienoic (roughanic) 16:3 |
| heptadecanoic 17:0 |
|||
| (_______________) stearic 18:0  |
(9-octadecenoic) oleic acid 18:1  |
(9,12-octadecadienoic) linoleic 18:2  |
(9,12,15-octadecatrienoic) linolenic acid 18:3 |
| nonadecanoic 19:0 |
|||
| (eicosanoic) arachidic 20:0  |
   |
(5,8,11,14-eicosatetraenoic) arachidonic 20:4 [  ] ] |
(5,8,11,14,17-eicosapentaenoic) timnodonic; EPA 20:5 |
| (docosanoic) behenic 22:0  |
(13-docosenoic) erucic 22:1 [  ] ] |
   |
(4,7,10,13,16,19-docosahexaenoic) cervonic; DHA 22:6 |
| (tetracosanoic) lignoceric 24:0  |
(15-tetracosenoic) nervonic 24:1 |
||
|
Fatty Acid Biosynthesis
The biosynthesis and metabolic function of polyunsaturated fatty acids (PUFAs) are usually dependent on the distance of the double bonds from the methyl or ω end of the molecule. Common polyunsaturated fatty acids are either ω-6 or ω-6 fatty acids. The double bonds of these fatty acids are also methylene (-C=C-C-C=C-) interrupted. Fatty acids with conjugated dienes (-C=C-C=C-) can have quite distinct properties. Cis unsaturated fatty acids have very different properties than trans unsaturated or saturated fatty acids. For example the melting point of stearic acid (18:0) is 70°C, oleic acid (cis 18:1) 6°C, linoleic acid (cis, cis 18:2) -5 °C and linolenic acid (cis, cis, cis 18:2) -11°C. Plants adapted for growth in temperate climates change the degree of unsaturation of membrane lipids in order to maintain membrane fluidity under different temperature conditions. The melting point of membrane lipids is determined by the combined fatty acid melting points. At temperatures below their melting points membranes are in the gel phase and in the liquid crystalline phase at temperatures above their melting points (see Jakubowski's online text for a model). Plants unable to rapidly increase desaturation of membrane fatty acids undergo chilling injury at moderately low temperatures (e.g. < ~10°C).
In plants primary saturated and monounsaturated fatty acids are made in plastids. Much of the polyunsaturated fatty acids are synthesized in the ER. Triglycerides accumulate in structures known as oil bodies. A general scheme of fatty acid biosynthesis in plant cells is depicted in the following figure:
KAS =
TE =
DS =
AT =
As mentioned above, synthesis of primary saturated and
monounsaturated fatty acid in plants occurs in plastids. In the first
step of fatty acid biosynthesis
acetyl-CoA + CO2  malonyl-CoA is catalyzed by acetyl-CoA carboxylase.
malonyl-CoA is catalyzed by acetyl-CoA carboxylase.
What is the source of the acetyl-CoA?
This reaction actually occurs in 2 steps and the acetyl-CoA carboxylase has 3 functional domains. The initial reaction involves an ATP-dependent transfer of CO2 from HCO3- to an N of the biotin prosthetic group of acetyl-CoA carboxylase. This activated CO2 is transferred from biotin to acetyl-CoA forming malonyl-CoA in the 2nd step. A model of the acetyl-CoA carboxylase complex is shown in Fig. 2 of Ohlrogge and Browse (1995) The Plant Cell 7, 957-970 and Figure 10.10 of the class text:
![[Image of acetyl-CoA carboxylase complex]](pcfig2.jpg)
The biotin, which "activates" the CO2, is attached to an 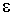 -amino of the biotin carboxyl carrier protein by the biotin carboxylase functional region. Carboxyltransferase transfers the
CO2 from biotin to acetyl-CoA forming malonyl-CoA. Plants
have been found to have 2 forms of acetyl-CoA carboxylase. A
tri-functional single protein > 200 kD and a multi-subunit heteromeric
complex. The multi-subunit form is found in plastids of most plants
except members of the Gramineae. Plastids and cytosol of grasses and
cytosol of other plants contain the single multi-functional polypeptide
form.
-amino of the biotin carboxyl carrier protein by the biotin carboxylase functional region. Carboxyltransferase transfers the
CO2 from biotin to acetyl-CoA forming malonyl-CoA. Plants
have been found to have 2 forms of acetyl-CoA carboxylase. A
tri-functional single protein > 200 kD and a multi-subunit heteromeric
complex. The multi-subunit form is found in plastids of most plants
except members of the Gramineae. Plastids and cytosol of grasses and
cytosol of other plants contain the single multi-functional polypeptide
form.
The synthesis of saturated fatty acids is generally similar in all organisms. However, fatty acid biosynthesis in plants has 2 fundamental differences from all other eukaryotic organisms. First, in plants this occurs in the stroma of plastids whereas in other eukaryotes it occurs in the cytosol. Second, saturated fatty acid synthesis, which requires at least 6 different enzymatic reactions, requires 6 different enzymes in plants in contrast to one hexa-functional protein in other higher eukaryotes (e.g. Fig 1 of Maier, Leibundgut and Ban, 2008).
Saturated fatty acid synthesis proceeds 2 carbon units per cycle usually 8 or 9
times producing 16 or 18 carbon products. The donor for the 2 carbon
units for each cycle is malonyl-ACP (acyl carrier protein) and the product
is also an ACP-thioester. Malonyl-ACP is formed from ACP and
malonyl-CoA by a transacylase. The next step of the fatty acid
synthesis cycle involves condensation of
malonyl-ACP + acetyl-CoA  3-ketobutyryl-ACP + CO2 + CoASH.
3-ketobutyryl-ACP + CO2 + CoASH.
This is driven by loss of CO2 making this pathway
unidirectional. The next step involves NADPH-dependent reduction of
the 3-ketobutyryl-ACP to 3-hydroxybutyryl-ACP. A dehydratase
subsequently dehydrates this to the unsaturated product trans-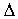 ²-butenoyl-ACP. The last step of the cycle is the
NADPH-dependent reduction of the double bond producing the 4C saturated
fatty acid thioester, butyryl-ACP. This cycle is repeated 7 or 8
times producing palmitoyl-ACP or stearoyl-ACP.
²-butenoyl-ACP. The last step of the cycle is the
NADPH-dependent reduction of the double bond producing the 4C saturated
fatty acid thioester, butyryl-ACP. This cycle is repeated 7 or 8
times producing palmitoyl-ACP or stearoyl-ACP.
The condensation reactions are catalyzed by enzymes known as 3-ketoacyl-ACP synthases or KASs. The 1st condensation reaction going from acetyl-CoA to 3-ketobutyrate is catalyzed by KAS III, the reaction from butyryl-ACP (C4) to palmitoyl-ACP (C16) by KAS I and from palmitoyl-ACP to stearoyl-ACP (C18) by KAS II. The saturated fatty acid synthesis cycle is illustrated in Fig. 3 of Ohlrogge and Browse (1995) The Plant Cell 7, 957-970 and Fig. 10.8 of the class text:
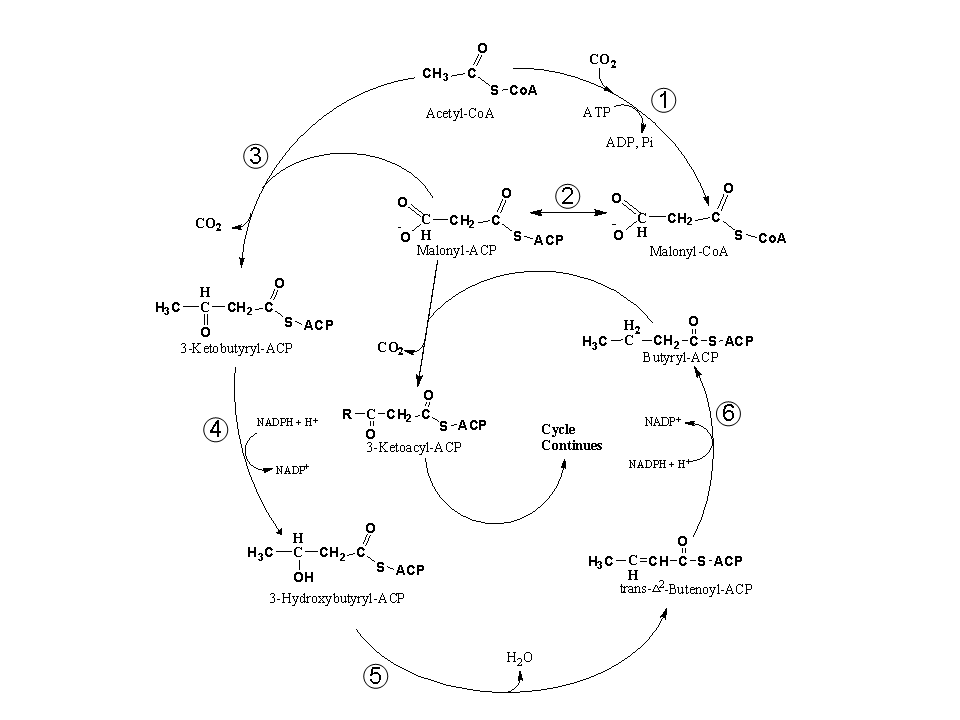
See pcfig3.jpg
Why is it that the reaction stops at C16 and C18 fatty acids?
The answer appears to be in the action of thioesterases (TEs) which hydrolyze the acyl-S-ACP thioester bonds. In other words TEs stop the reaction at the appropriate chain length. Some plants have unusual TEs, known as medium chain TEs, which stop the reaction at C8, C10, C12 or C14 fatty acid chain lengths.
Once a TE releases the fatty acids, they are either incorporated into plastid glycerolipids by acyltransferases (ATs) or they are transferred to the cytosol and esterified to CoA. This is illustrated in the following figure:
Saturated Fatty Acid Thioesterase genes are known as FatBs and that for the predominate unsaturated fatty acid-ACP synthesized in plastids FatA:
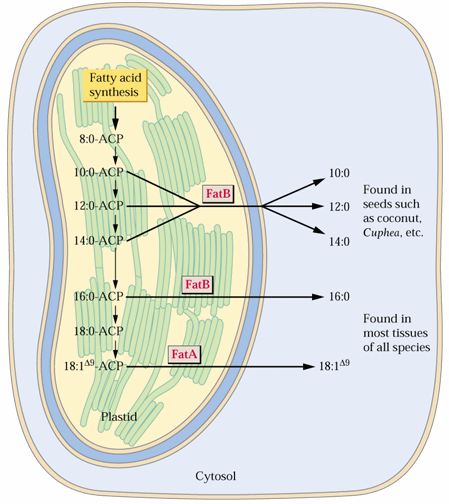
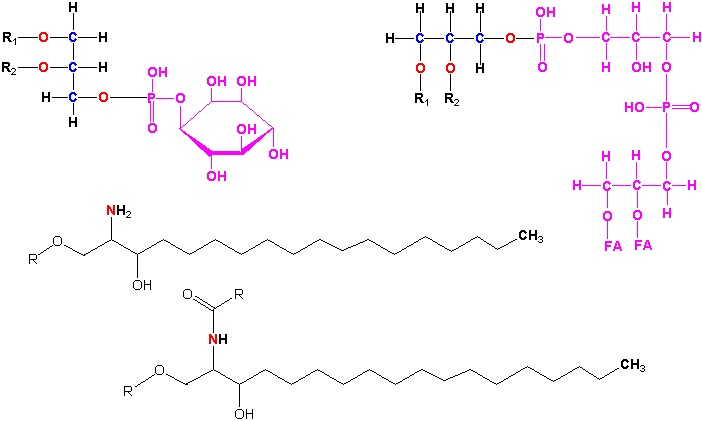
The fatty acids transferred to the cytoplasm enter the acyl-CoA pool providing fatty acids for glycerolipid synthesis (see lecture 15) or further elongation in the cytosol. The further elongated fatty acids (C20 to ~C32), known as very long chain fatty acids (VLCFAs), can occasionally be put into triacylglycerol or used in synthesis of wax, cutin or suberin.
Wax synthesis
In wax synthesis some of the VLCFAs (as CoA thioesters), e.g. C30 and C32 are decarboxylated into C29 and C31 pure hydrocarbons. Some of these long chain hydrocarbons are hydroxylated in the middle to alcohols, some of which can be further oxidized to ketones. Others of the VLCFAs have the carboxylic acid reduced to aldehydes, some of which are further reduced to a-hydroxy alcohols. The a-hydroxy alcohols can be esterified to VLCFA-CoAs forming esters usually 44-52 carbons long. All 7 of these fatty acid derivatives are components of wax. Wax synthesis is illustrated in the following figure:
![[Image
of wax synthesis]](wax.gif)
Cutin biosynthesis
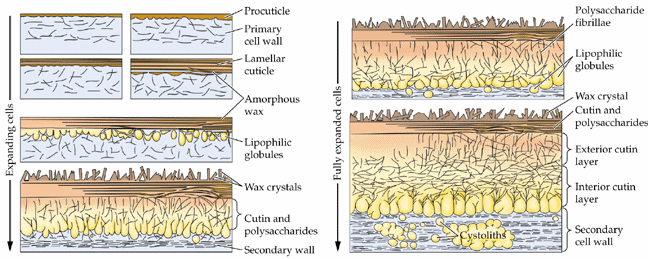
Cutin synthesis, the other major cuticular component, involves modification of some palmitoyl- and oleoyl-CoAs to hydroxy, epoxy and dihydroxy derivatives all of which (~8 monomers) are inter-esterified to form complex polyesters. Suberin biosynthesis is similar but we do not have time to cover it here. Cutin synthesis is illustrated in the following figures:
![[Image of cutin synthesis, part 1]](C16-C18.gif)
![[Image of cutin synthesis, part 2]](fig8-26.gif)
Cutin and wax biosynthetic pathways - Fig. 2 of Yeats & Rose (2013)
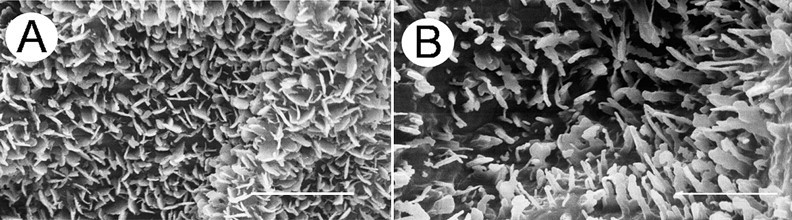
Wax crystals on the surface of pea leaves (Gniwotta et al., 2005; Fig. 3):
Regulation of cuticle biosynthesis - Fig. 4 of Yeats & Rose (2013)
Reading Assignment for the second lipid lecture:
a) REQUIRED:
1 - Shimada, T.L., Hayashi, M., and Hara-Nishimura, I. (2018). Membrane Dynamics and Multiple Functions of Oil Bodies in Seeds and Leaves. Plant Physiology 176, 199-207.
2 - Bates, P.D., Fatihi, A., Snapp, A.R., Carlsson, A.S., Browse, J., and Lu, C. (2012). Acyl Editing and Headgroup Exchange Are the Major Mechanisms That Direct Polyunsaturated Fatty Acid Flux into Triacylglycerols. Plant Physiology 160, 1530-1539.
3 - Chapter 10, sections 10.5 - 10.8 and 10.10.1 -10.10.3, of the Biochemistry & Molecular Biology of Plants class text.
b) OPTIONAL:
1 - Lager, I., Yilmaz, J.L., Zhou, X.-R., Jasieniecka, K., Kazachkov, M., Wang, P., Zou, J., Weselake, R., Smith, M.A., Bayon, S., et al. (2013). Plant Acyl-CoA:Lysophosphatidylcholine Acyltransferases (LPCATs) Have Different Specificities in Their Forward and Reverse Reactions. Journal of Biological Chemistry 288, 36902-36914.
2 - Zhang, M., J. Fan, D.C. Taylor, and J.B. Ohlrogge. 2009. DGAT1 and PDAT1 Acyltransferases Have Overlapping Functions in Arabidopsis Triacylglycerol Biosynthesis and Are Essential for Normal Pollen and Seed Development. Plant Cell 21:3885-3901.
3 - Engelman, D.M. 2005. Membranes are more mosaic than fluid. Nature 438: 578-580.
4 - Lu, C., Z. Xin, Z. Ren, M. Miquel, and J. Browse. 2009. An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proceedings of the National Academy of Sciences 106:18837-18842.
5 - McMahon, H.T., and J.L. Gallop. 2005. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438:590-596.
6 - Chapman K.D., Ohlrogge J.B. (2012) Compartmentation of Triacylglycerol Accumulation in Plants. Journal of Biological Chemistry 287:2288-2294.
| All materials © 2018 David Hildebrand, unless otherwise noted. | |||||||
| home | syllabus | lecture schedule & web notes | virtual office hours | messages & answers from the instructor | supplementary material | related links | What's new? |