
Lecture Twenty-three
Nitrogen Metabolism -- nitrate reduction, ammonia assimilation

Goal: A good working knowledge and understanding of the reduction of oxidized forms of nitrogen commonly encountered by plants to ammonia and the assimilation of the reduced ammonia into major nitrogenous compounds of plant tissues.
Outline:
Background Readings for the discussion on nitrate reduction and ammonia assimilation :
1 - Chapter 16, sections 16.6 - 16.10 of the Biochemistry & Molecular Biology of Plants class text.
2 - Han, Y.-L., Song, H.-X., Liao, Q., Yu, Y., Jian, S.-F., Lepo, J.E., Liu, Q., Rong, X.-M., Tian, C., Zeng, J., et al. (2016). Nitrogen use efficiency is mediated by vacuolar nitrate sequestration capacity in roots of Brassica napus. Plant Physiology 170:1684-1698.
b) SUGGESTED:
1 - Lee, J.-H., B.S. Evans, G. Li, N.L. Kelleher, and W.A. van der Donk. 2009. In Vitro Characterization of a Heterologously Expressed Nonribosomal Peptide Synthetase Involved in Phosphinothricin Tripeptide Biosynthesis. Biochemistry 48:5054-5056..
2 - Crawford, Nigel M. 1995. Nitrate: Nutrient and signal for plant growth. The Plant Cell 7: 859-868.
3 - Scheible, W.-R., R. Morcuende, T. Czechowski, C. Fritz, D. Osuna, N. Palacios-Rojas, D. Schindelasch, O. Thimm, M.K. Udvardi, and M. Stitt. 2004. Genome-Wide Reprogramming of Primary and Secondary Metabolism, Protein Synthesis, Cellular Growth Processes, and the Regulatory Infrastructure of Arabidopsis in Response to Nitrogen. Plant Physiol. 136: 2483-2499.
4 - Forde, B.G. 2002. LOCAL AND LONG-RANGE SIGNALING PATHWAYS REGULATING PLANT RESPONSES TO NITRATE. Annu. Rev. Plant Biol. 53: 203-24.
5 - Lea, U.S., M.-T. Leydecker, I. Quillere, C. Meyer, and C. Lillo. 2006. Posttranslational Regulation of Nitrate Reductase Strongly Affects the Levels of Free Amino Acids and Nitrate, whereas Transcriptional Regulation Has Only Minor Influence. Plant Physiol. 140: 1085-1094.
6 - Okamoto, M., A. Kumar, W. Li, Y. Wang, M.Y. Siddiqi, N.M. Crawford, and A.D.M. Glass. 2006. High-Affinity Nitrate Transport in Roots of Arabidopsis Depends on Expression of the NAR2-Like Gene AtNRT3.1. Plant Physiol. 140:1036-1046.
7- Remans, T., P. Nacry, M. Pervent, T. Girin, P. Tillard, M. Lepetit, and A. Gojon. 2006. A Central Role for the Nitrate Transporter NRT2.1 in the Integrated Morphological and Physiological Responses of the Root System to Nitrogen Limitation in Arabidopsis. Plant Physiol. 140:909-921
Remember from the previous class discussion, nitrogen (N2) must first be fixed usually into a reduced form such as ammonia. Ammonia is usually rapidly oxidized into nitrate by nitrifying bacteria in soils so nitrate is the usual form of nitrogen available to most plants.
 |  |  | ||
 |  |  |
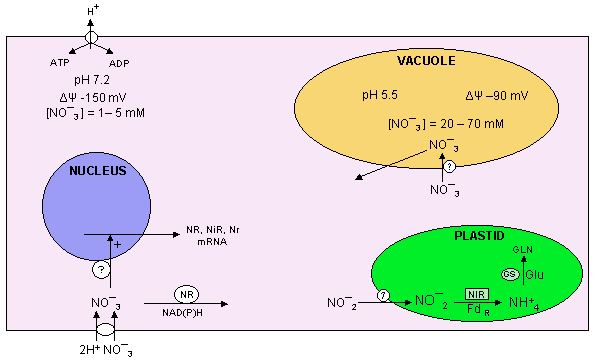
 ) gradients.
) gradients.

[not covered in 2017]
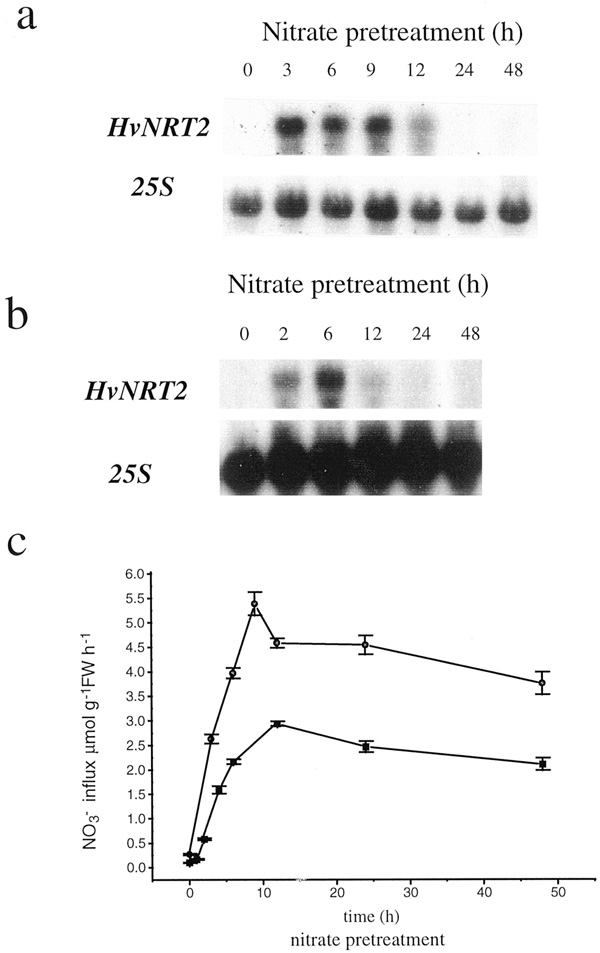
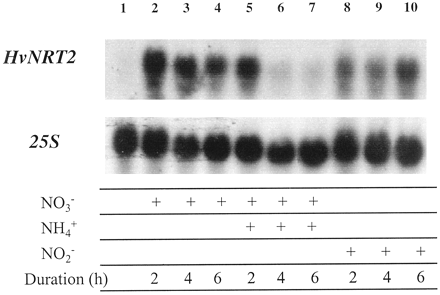
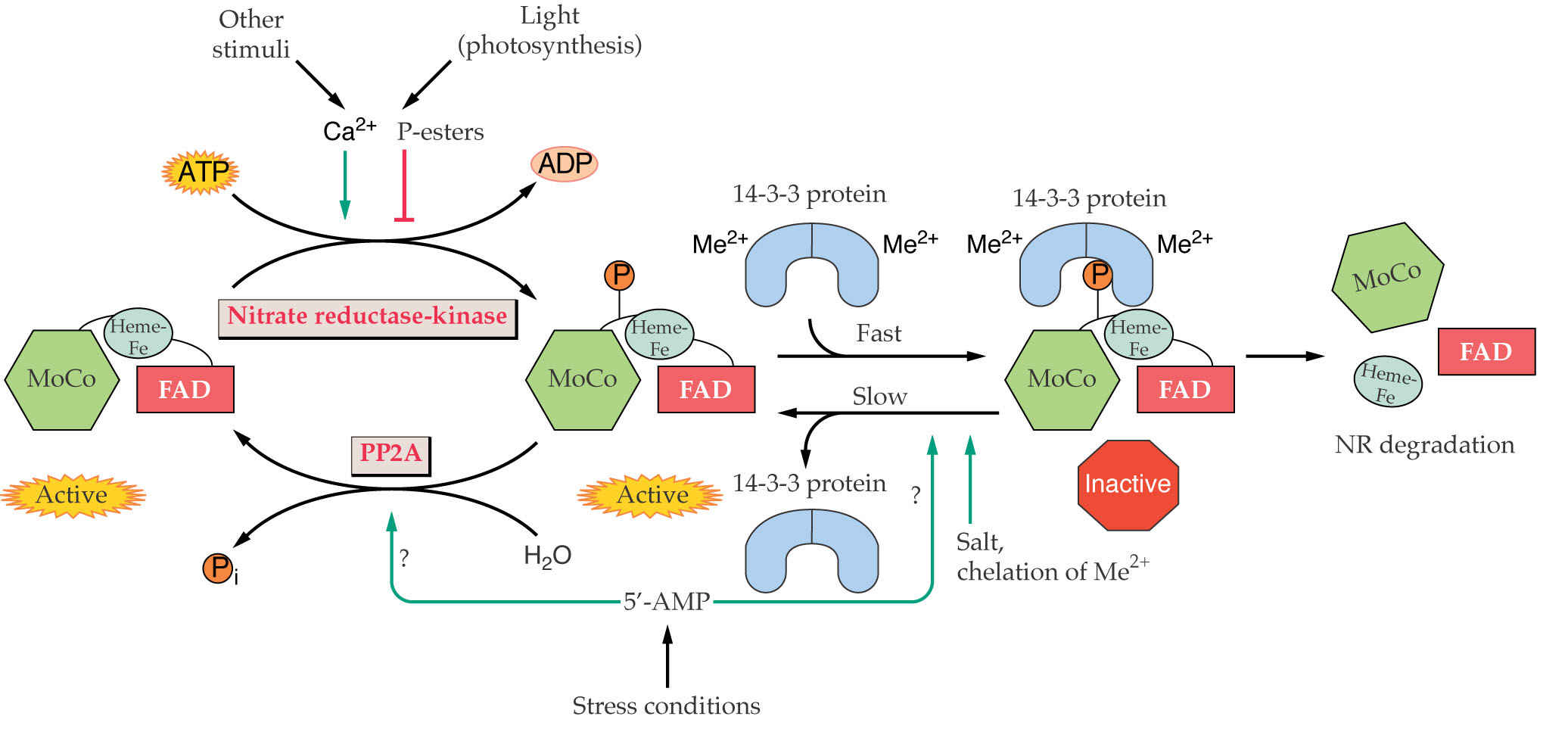
Not only is the nitrate uptake system induced, but also nitrate induces nitrate and nitrite reductase activities by altering gene expression mainly by enhancing transcription of the respective genes. Nitrate, light and sugars are inducers; glutamate and glutamine are repressors.
[covered in 2017]
 NO2- +
H2O
NO2- +
H2O NH4+ +
2H2O
NH4+ +
2H2O
[not covered in 2017]
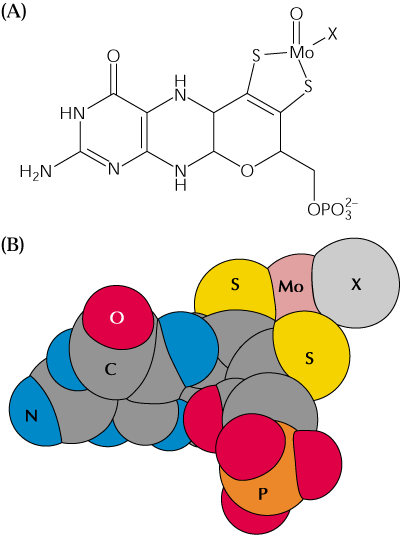
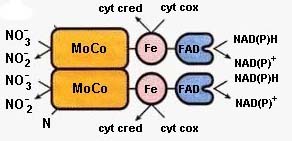
The NR catalyzed reduction of NO3- starts with e- transport from NAD(P)H to the flavin domain, through heme and finally onto NO3- via the molybdenum cofactor. Cytochrome c can be an alternative e- acceptor (Fig. 2, 16.38 above).
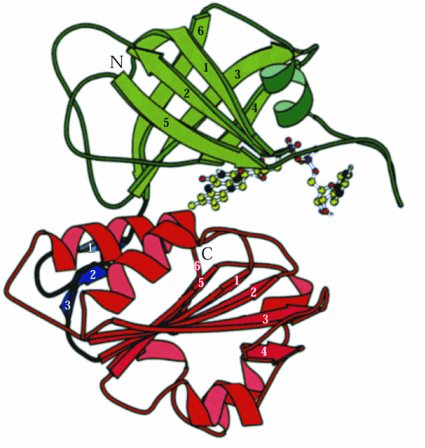
The central heme containing 75-80 amino acids is similar to heme of cytochrome b5s. See illustration at the Directory of P450-containing Systems web page.
[end not covered 2017]
Green tissues have >>> NR than NiR activity so NR is rate limiting. What might be the advantages of this?
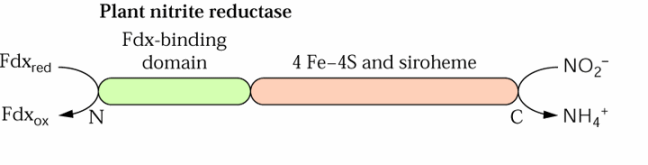

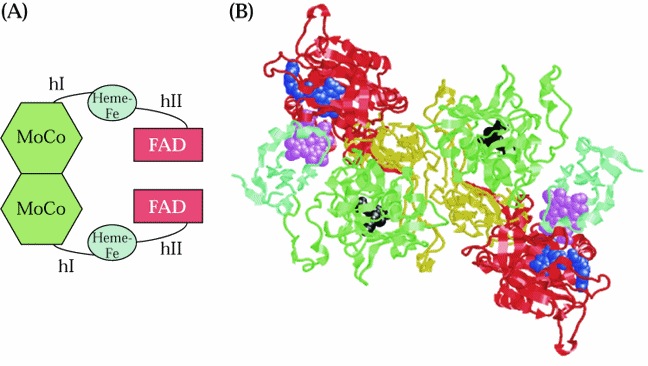
The c-terminal half of NiR is thought to contain the redox centers and the N-terminal half is thought to bind the reducing agent ferredoxin.
NH4+ + HCO3- + 2ATP
 H2N-CO-O-PO3-2 + 2ADP + Pi
H2N-CO-O-PO3-2 + 2ADP + Pi
GDH has a significantly higher km for NH4+ than does glutamine synthetase (GS). Consequently in organisms confronting N-limitation GDH is not effective and GS is the only NH4+ assimilation reaction. It also appears that GS is the sole port of entry of N into amino acids.
The reaction: Glutamate + ammonia + ATP  glutamine + ADP + Pi
glutamine + ADP + Pi
involves activation of the g-carboxyl group of Glu by ATP followed by amination by
NH4+:
 |
|
 2Glu + oxidized
reductant
2Glu + oxidized
reductant
 Glutamine + 2 Fdox + 2 ADP +
2 Pi
Glutamine + 2 Fdox + 2 ADP +
2 Pi
ADP glucose pyrophosphorylase ↓↓
Scheible et al. (2004) examined the genome-wide metabolic responses of Arabidopsis to N. Numerous overall metabolic changes after transfer of N-deficient Arabidopsis seedlings to NO3- sufficient medium after 30 min.:
and after 3h:
Background Readings for the discussion on amino acid and other N compound metabolism :
a) REQUIRED:
1 - Chapter 16, sections 16.11 - 16.18 of the Biochemistry & Molecular Biology of Plants class text.
b) SUGGESTED:
1 - Lam et al. 1995. Use of Arabidopsis mutants and genes to study amide amino acid biosynthesis. The Plant Cell 7, pp. 887-898.
2 - Guo, F.Q., M. Okamoto and N.M. Crawford. 2003. Identification of a plant Nitric oxide synthase gene involved in hormone signaling. Science 302: 100-103.
3 - Shewry et al. 1995. Seed storage proteins: structures and biosynthesis. The Plant Cell 7, pp. 945-956.
4 - von Wettstein et al. 1995. Chlorophyll biosynthesis. The Plant Cell 7, pp. 1039-1057.
5 - Zeier, J., M. Delledonne, T. Mishina, E. Severi, M. Sonoda, and C. Lamb. 2004. Genetic Elucidation of Nitric Oxide Signaling in Incompatible Plant-Pathogen Interactions. Plant Physiol. 136: 2875-2886.
6 - Jasid, S., M. Simontacchi, C.G. Bartoli, and S. Puntarulo. 2006. Chloroplasts as a Nitric Oxide Cellular Source. Effect of Reactive Nitrogen Species on Chloroplastic Lipids and Proteins. Plant Physiol. 142:1246-1255.
7 - Peng Pan, Eilika Woehl and Michael F. Dunn. 1997. Protein architecture, dynamics and allostery in tryptophan synthase channeling. TIBS 22: 22-27.
| All materials © 2017 David Hildebrand, unless otherwise noted. | |||||||
| home | syllabus | lecture schedule & web notes | virtual office hours | messages & answers from the instructor | supplementary material | related links | What's new? |