
Lecture Twenty-five
Nitrogen Metabolism -- Sulfur
Reduction and Assimilation; N/Sulfur Compounds
Goal: Provide a fundamental understanding of sulfur reduction, assimilation
and metabolism in plants. Discussion of selected important sulfur compounds of
plant tissues.
Outline:
- Introduction
- Sulfur reduction
- Sulfur assimilation
- Sulfur metabolism
- N/Sulfur compounds
Background Readings for the discussion on N/S compounds :
a) REQUIRED:
1 - Chapter 16 sections 16.11 - 16.18 (16.10 - 16.14 2000 version) of the Biochemistry & Molecular Biology of Plants class text.
b) SUGGESTED:
1 - Stephane
Ravenel, Bertrand Gakiere, Dominique Job and Roland Douce. 1998. The specific
features of methionine biosynthesis and metabolism in plants. PNAS
95: 7805-7812.
2 - Thomas Leustek,
Melinda N. Martin, Julie-Ann Bick and John P. Davies. 2000. PATHWAYS AND
REGULATION OF SULFUR METABOLISM REVEALED THROUGH MOLECULAR AND GENETIC STUDIES.
Annu. Rev. Plant Physiol. Plant. Mol. Biol. 51:
141-165.
3 - Naoko Yoshimoto,
Eri Inoue, Kazuki Saito, Tomoyuki Yamaya and Hideki Takahashi. 2003. Phloem-Localizing
Sulfate Transporter, Sultr1;3, Mediates Re-Distribution of Sulfur from Source
to Sink Organs in Arabidopsis1. Plant Physiol. 131: 1511-1517.
4 - Kopriva, S., M.
Suter, P. von Ballmoos, H. Hesse, U. Krahenbuhl, H. Rennenberg, and C. Brunold.
2002. Interaction of Sulfate Assimilation with Carbon and Nitrogen Metabolism
in Lemna minor. Plant Physiology. 130: 1406-1413.
5 - Anna Koprivova, Marianne
Suter, Roel Op den Camp, Christian Brunold, Stanislav Kopriva. 2000. Regulation
of Sulfate Assimilation by Nitrogen in Arabidopsis. Plant Physiol. 122:
737-746.
6 - Shibagaki, N.,
A. Rose, J.P. McDermott, T. Fujiwara, H. Hayashi, T. Yoneyama and J.P. Davies.
2002. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a
sulfate transporter required for efficient transport of sulfate into roots.
Plant J. 29:
475-486.
About 70% of the organic sulfur in plants is associated with the 2 sulfur
containing amino acids in proteins, cysteine and methionine. The
remaining 30% involves soluble amino acids and peptides, mostly
glutathione. About 1% of the organic sulfur in plants is a component of
sulfolipid.
Like nitrogen, sulfur can exist in multiple oxidation states:
|
|
+4
|
+2
|
0
|
|
|
SO4-2
|
SO3-2
|
S2O3-2
|
S
|
H2S
|
Sulfur Reduction
Plants usually take up sulfur in its oxidized form, sulfate, and as the case
with nitrate the sulfate must 1st be reduced before incorporation
into organic molecules. Sulfate reduction begins with internalization of
sulfate by a high affinity uptake mechanism. Sulfate is transported into
chloroplasts by the triose-P translocator in exchange for phosphate. Once
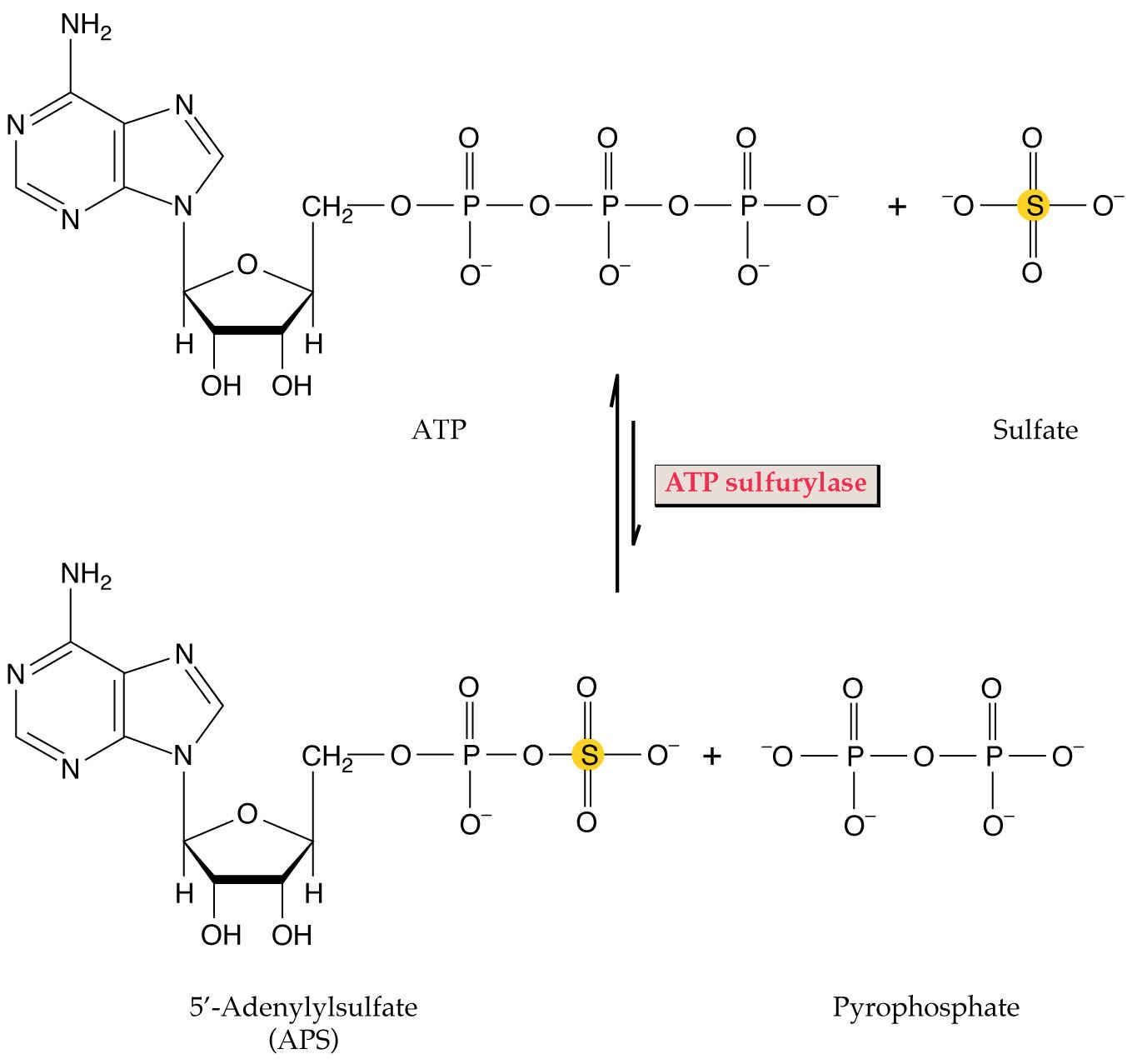
inside chloroplasts, sulfate reduction begins with activation in the presence
of ATP forming adenosine phosphosulphate (APS) also called AMP sulfate
(see Fig. 2 of Leustek and Saito, 1999):
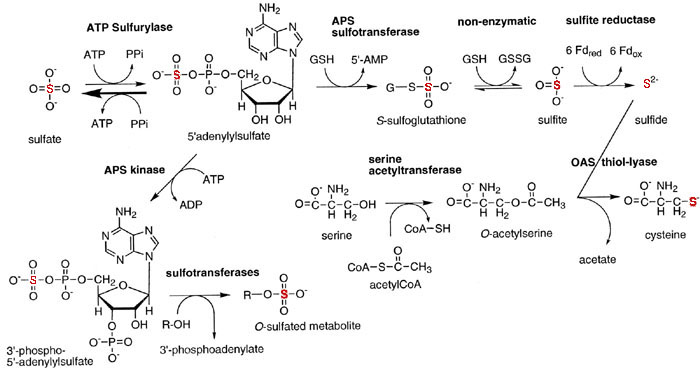
MgATP + sulfate  APS + MgPPi
APS + MgPPi
The PPi  2Pi helping drive this reaction forward.
2Pi helping drive this reaction forward.
APS is further phosphorylated by APS kinase producing P-APS. In this form the sulfate of P-APS is reduced to sulfite by the free thiol (reduced) group of
thioredoxin. Thioredoxin can also reduce disulfide bonds in proteins to free thiol groups:
Sulfur Assimilation
The reduced sulfur is finally transferred from the carrier to O-acetylserine (OAS) forming cysteine:
O-acetylserine + H2S (or carrier-S-S-)  cysteine + acetate
cysteine + acetate
The OAS is formed from serine and acetyl-CoA:
serine + acetyl-CoA  O-acetylserine + CoA
O-acetylserine + CoA
This whole process is illustrated as follows:
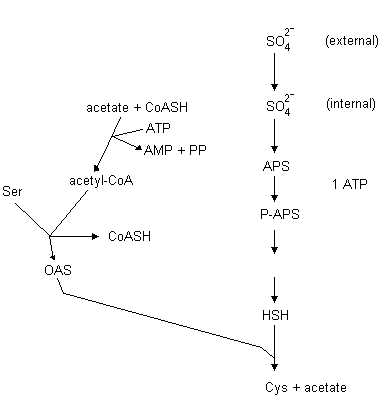
|
This is summarized in Fig. 16.45 (PPt):
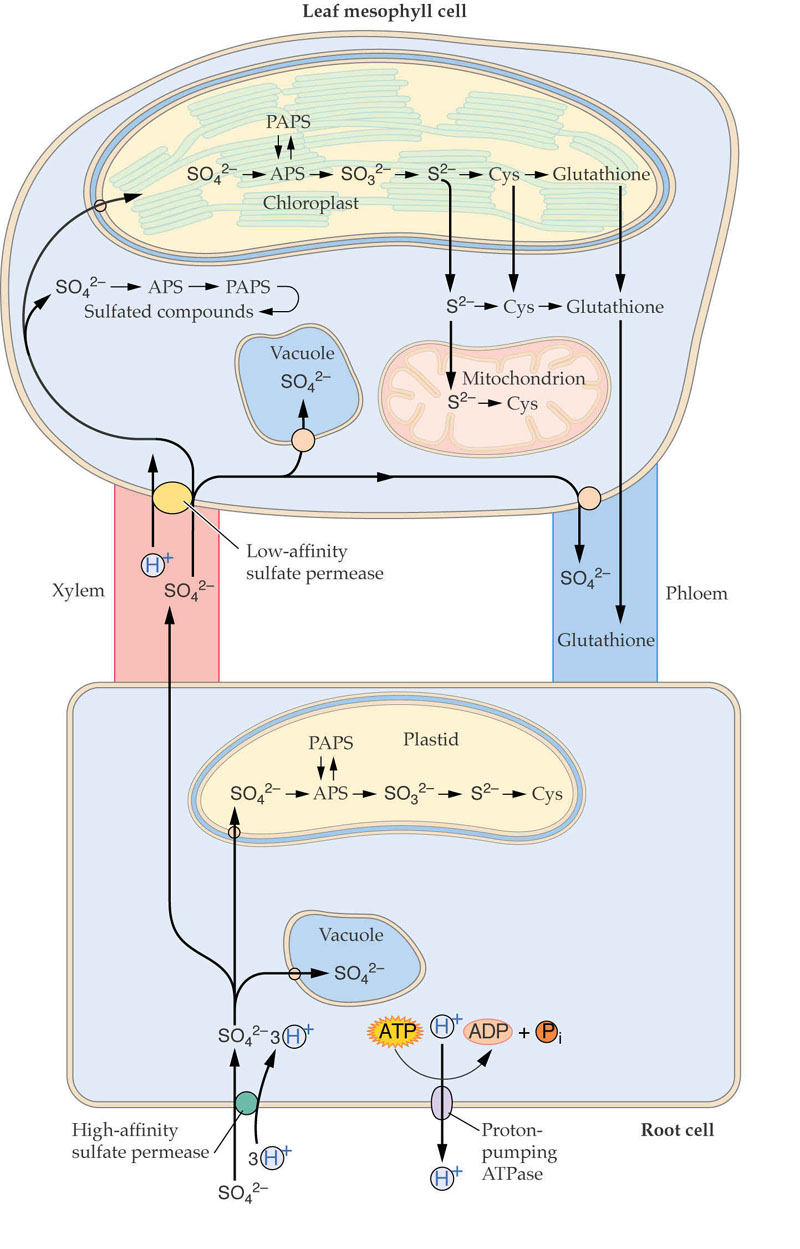
Sulfur Metabolism
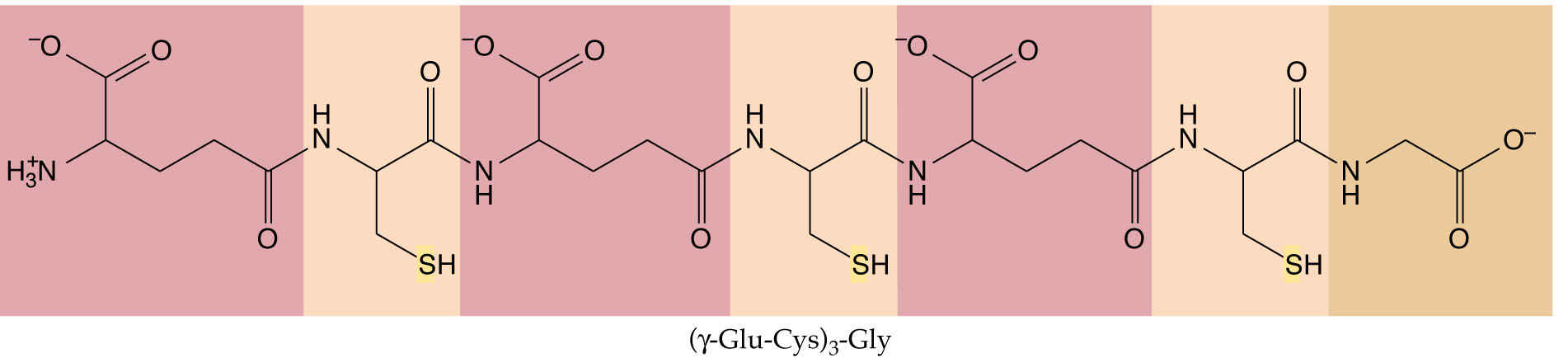
Very little cysteine is found in vivo. Instead it is converted to methionine
upon reaction with phosphohomoserine or is rapidly converted to the tripeptide glutathione
= glutamate-cysteine-glycine (ECG). Glutathione, ascorbate and superoxide
dismutase (SOD) are key components in plants (especially in plastids) for the detoxification
of active oxygen species such as superoxide (O2-).
SOD converts superoxide to hydrogen peroxide:
2O2- + 2H+  H2O2
+ O2
H2O2
+ O2
The hydrogen peroxide is detoxified by the combined action of ascorbate
peroxidase, dehydroascorbate reductase and glutathione reductase as mentioned
in Lecture 17:
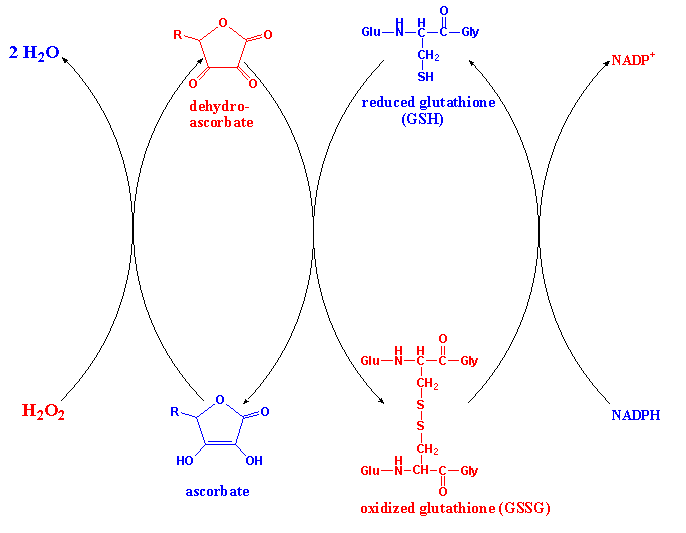
Many other toxic substances taken in by organisms from the environment (xenobiotics)
are detoxified by reaction with glutathione. The reactive SH group of
glutathione can form a thioether by reacting with C=C double bonds, carbonyl
groups or other reactive groups forming a glutathione conjugate. The
formation of glutathione conjugates is catalyzed by glutathione-S-transferases
(GSH). Such glutathione conjugates are actively transported into vacuoles in an
ATP-dependent process for detoxification. Crop safeners promote this
process of herbicide detoxification:
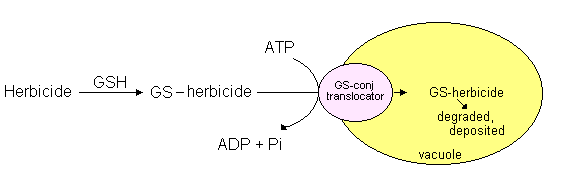
Phytochelatins derived from glutathione protect against toxic heavy metals:
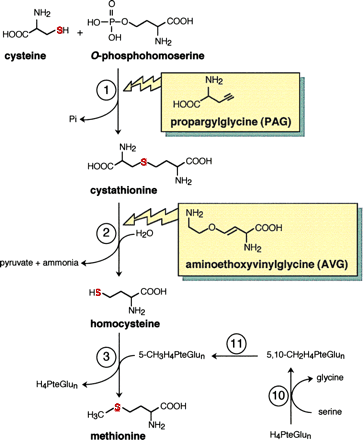
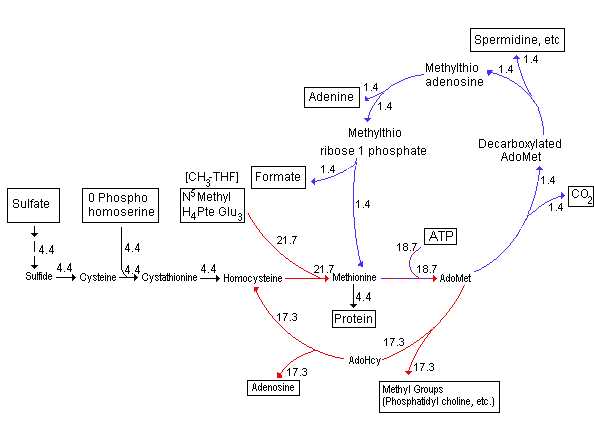
Cysteine provides the sulfur for methionine in a process known as
transsulfuration. In methionine synthesis cysteine reacts with the C4
skeleton of phosphohomoserine (PHS) to form cystathionine followed by removal
of the original C3 skeleton of cysteine resulting in homocysteine.
Transfer of a methyl group from methyltetrahydrofolate (CH3-THF)
by methionine synthase then forms methionine. Methionine can be converted
into S-adenosylmethionine (SAM) by SAM synthetase. Sulfate
reduction and cysteine, cystathionine and homocysteine syntheses occur in
chloroplasts. The homocysteine (and cystathionine in some cases) is
transferred to the cytoplasm where methionine and SAM are formed (Fig. 6,
Anderson, 1990; Fig. 1 Ravanel
et al., 1998):
Folates are important acceptors
and donors of 1-C units for all oxidation states of C except CO2
(where biotin is the relevant carrier).
folate + 4H+  THF
THF
CH3-THF:
About 4 times as much methionine
cycles through SAM as a methyl donor in the synthesis of numerous other
molecules in plant cells vs. protein synthesis. Of these methylated ethanolamine derivatives
such as the choline of phosphatidylcholine followed by pectins are
quantitatively most important. Some of the SAM is used in polyamine
synthesis as mentioned in Lecture 24. In these reactions the reduced
sulfur is recycled back into methionine as illustrated:
[skip for 2017]
N/Sulfur Compounds
The L-asparagine analog, L-3-cyanoalanine, is synthesized from L-cysteine +
HCN.
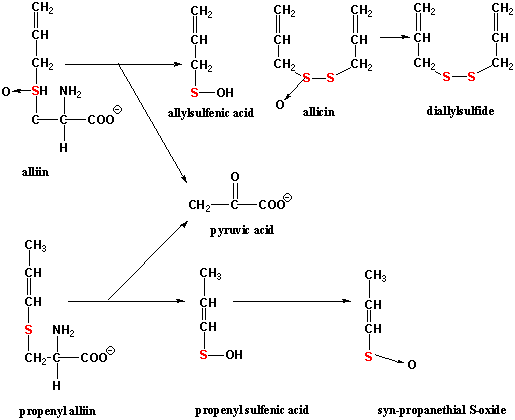
An interesting group of cysteine derivatives found in garlic and onions, Allium
cepa, A. sativum and A. ursinum, are alliin and
derivatives. Alliin and propenylallin are hydrolyzed (cleaved) to
allylsulfenic acid, propenyl sulfenic acid and pyruvic acid + NH3 by
alliin lyase when garlic or onion tissues are damaged. Allylsulfenic acid
spontaneously condenses to the disulfides, allicin and to dialylsulfide.
Allicin and dialylsulfide have strong antimicrobial activity. Propenyl
sulfenic acid spontaneously rearranges to syn-propanethial
S-oxide. Allicin and syn-propanethial S-oxide have strong feeding
deterrent activity toward herbivores such as insects. syn-Propanethial
S-oxide is a lachrymator -- the reason why slicing onions causes tears to form:
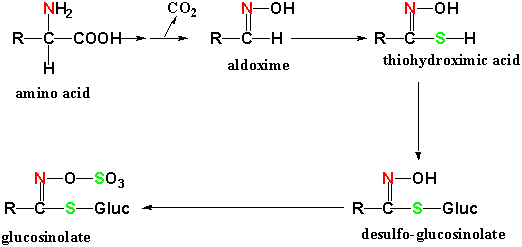
Certain plants in the dicot order
Capparales such as in the Cruciferae accumulate thioglucosides known as glucosinolates in vacuoles that are similar to cyanogens in many aspects. All plant
cells that sequester glucosinolates in vacuoles also have thioglucoside
glucohydrolases known as myrosinase stored in places other than
vacuoles. Upon tissue damage or wounding or large increases in membrane
permeability, myrosinase and glucosinolates come together releasing the
pungent, repellent and microbicidal compound, isothiocyanate:
The distinctive, pungent flavor and odor of mustard,
radishes and horseradish is due to isothiocyanates. Glucosinolates are
synthesized from various protein and non-protein amino acids, cysteine and
glucose in a manner very similar to the biosynthesis of cyanogenic glucosides
except for the addition of sulfur from cysteine. The precursor amino acid
is converted to an aldoximine and to thiohydroximic acid after addition of a
sulfate group. A thioglucoside, desulfo-glucosinolate, is formed at the
next step with addition of glucose from UDP-glucose. Finally another
sulfur group is added in the form of sulfate by transfer from phosphoadenosine-phosphosulfate
(PAPS) forming a glucosinolate:
Other
members of the cabbage family including cabbage itself produce some level of
isothiocyanates. Concentrations of up to 8% are seen in seeds of some
Cruciferae. One of the few developments of a major new crop in modern
times is the development of canola from Brassica napus rapeseed.
Although high levels of oil and protein meal can be produced in cool areas, the
use of traditional rapeseed has been limited. The oil was not suitable
for edible uses because of high levels of erucic acid, which causes cardiac
lesions. The erucic acid levels were not high enough for high value as an
industrial oil. The meal was not suitable as an animal feed because of
high glucosinolate levels. Large reductions in erucic acid and
glucosinolate levels of certain rapeseed genotypes led to the development of
canola by Canadian plant breeders. In the last 30 years canola has risen
to be one of the major crops of the world especially in
Canada
.
Background Readings for the discussion on alkaloids:
a) REQUIRED:
1 - Schardl, C. L., R. B.
Grossman, P. Nagabhyru, J. R. Faulkner and U. P. Mallik. 2007. "Loline
alkaloids: Currencies of mutualism." Phytochemistry
68(7): 980-996.
2 - Chapter 24 sections 24.6 - 24.8 of the
Biochemistry & Molecular Biology of Plants class text.
b) SUGGESTED:
1 - Schardl, C. L., D. G. Panaccione
and P. Tudzynski. 2007. Ergot alkaloids -- Biology and molecular biology. The
Alkaloids: Chemistry and Biology 63:
45-86.
2 - Rudgers, J.A., S. Fischer, and K. Clay. 2010. Managing plant symbiosis: fungal endophyte genotype alters plant community composition. Journal of Applied Ecology 47:468-477.
2 - Peter J. Facchini. 2001.
ALKALOID BIOSYNTHESIS IN PLANTS: Biochemistry, Cell Biology, Molecular
Regulation, and Metabolic Engineering Applications. Annu. Rev. Plant Physiol.
Plant. Mol. Biol. 52:
29-66.
3 - Sato, Fumihiko, Takashi
Hashimoto, Akira Hachiya, Ken-ichi Tamura, Kum-Boo Choi, Takashi Morishige,
Hideki Fujimoto and Yasuyuki Yamada. 2001. Metabolic engineering of plant
alkaloid biosynthesis. PNAS 98:
367-372.
4 - Misako Kato,
Kouichi Mizuno, Alan Crozier, Tatsuhito Fujimura, and Hiroshi Ashihara. 2000.
Caffeine synthase gene from tea leaves. Nature 406: 956-957.
5 - André Kessler
and Ian T. Baldwin. 2002. PLANT RESPONSES TO INSECT HERBIVOR: The Emerging
Molecular Analysis. Annu. Rev. Plant Biol. 53:
299-328.
Please e-mail us if
you have any questions or comments regarding the class or the webpages.
This page was last modified Feb. 10, 2017.


![]() APS + MgPPi
APS + MgPPi![]() 2Pi helping drive this reaction forward.
2Pi helping drive this reaction forward.
![]() cysteine + acetate
cysteine + acetate![]() O-acetylserine + CoA
O-acetylserine + CoA








